Real-World Clinical Data of Palbociclib in Asian Metastatic Breast Cancer Patients: Experiences from Eight Institutions
Article information
Abstract
Purpose
Use of cyclin-dependent kinase 4/6 inhibitors improved survival outcome of hormone receptor (HR) positive metastatic breast cancer (MBC) patients, including Asian population. However, Asian real-world data of palbociclib is limited. We analyzed the real-world clinical practice patterns and outcome in HR-positive, MBC Asian patients treated with palbociclib.
Materials and Methods
Between April 2017 to November 2019, 169 HR-positive, human epidermal growth factor-2–negative MBC patients treated with letrozole or fulvestrant plus palbocilib were enrolled from eight institutions. Survival outcome (progression-free survival [PFS]), treatment response and toxicity profiles were analyzed.
Results
Median age of letrozole plus palbociclib (145 patients, 85.8%) and fulvestrant plus palbociclib (24 patients, 14.2%) was 58 and 53.5 years, with median follow-up duration of 14.63 months (range 0.2 to 33.9 months). Median PFS (mPFS) of letrozole plus palbociclib and fulvestrant plus palbociclib was 25.6 (95% confidence interval [CI], 19.1 to not reached) and 6.37 months (95% CI, 5.33 to not reached), comparable to previous phase 3 trials. In letrozole plus palbociclib arm, luminal A (hazard ratio, 2.86; 95% CI, 1.20 to 6.80; p=0.017) and patients with good performance (Eastern Cooperative Oncology Group 0–1 [hazard ratio, 3.68; 95% CI, 1.70 to 7.96]) showed better mPFS. In fulvestrant plus palbociclib group, chemotherapy naïve patients showed better mPFS (hazard ratio, 12.51, 95% CI, 1.59 to 99.17; p=0.017). The most common grade 3 or 4 adverse event was neutropenia (letrozole 86.3%, fulvestrant 88.3%).
Conclusion
To our knowledge, this is the first real-world data of palbociclib reported in Asia. Palbociclib showed comparable benefit to previous phase 3 trials in Asian patients during daily clinical practice.
Introduction
Breast cancer is the most common cancer diagnosed in women worldwide [1], and also the most common cancer in Korean women [2]. Hormone receptor (HR)–positive breast cancer accounts 70% of breast cancer with median age of 60–70 years in Western countries [3,4]. The incidence of HR-positive breast cancer in Asian countries is similar to Western [5], but the peak incidence of breast cancer in Asian countries is at around 45 to 50 years of age, relatively younger compared to Western countries [6]. Furthermore, incidence of breast cancer in young age population in some Asian countries even exceed that of United States in recent generation [7]. Therefore, higher proportion of patients are premenopausal in Asian HR-positive breast cancer compared to Western population. Other than age population and menopausal status, tumor biology and molecular signature may differ between Western and Asian patient population [8,9].
Endocrine treatment is the cornerstone of treatment in HR-positive metastatic breast cancer (MBC) and combination of cyclin-dependent kinase 4/6 (CDK4/6) inhibitor with letrozole or fulvestrant significantly improved clinical outcomes including overall survival in HR-positive MBC [10–15], changing the landscape of treatment paradigm. Patient population in previous phase III trials mostly reflected Western population with higher proportion of postmenopausal women. Subgroup analyses with Asian patient population reported similar survival outcome and quality of life, but with more hematologic toxicity [16,17]. However, only 10% to 30% of patients were Asian subpopulation in pivotal phase III trials, relatively under-represented during analysis. Furthermore, phase III trials covering first-line endocrine therapy plus CDK4/6 inhibitor [10,11,14] enrolled postmenopausal patients only, except MONALEESA-7 [18]. PALOMA-3 and MONARCH-2 trials enrolled premenopausal patients, but those proportion was relatively small, taking 17% to 21% of total patient population [13,15]. Premenopausal women take considerable patient population in recurrent, MBC, but these patients were consistently under-represented in pivotal trials. There are growing need of clinical trials for Asian patients and premenopausal HR-positive, human epidermal growth factor-2 (HER2)–negative breast cancer patients [19]. Until pivotal phase III trial including Asian and premenopausal women is presented, treatment guidelines are extrapolated from data from Western, postmenopausal women. In this situation, real-world data of Asian subpopulation may act as a reference during patients’ treatment.
Palbociclib was approved by U.S. Food and Drug Administration in February 2015, and also approved by Korea Food and Drug Administration in August 2016. After its approval, palbociclib is the most widely used CDK4/6 inhibitor worldwide including Korea. However, there are few data reporting the treatment pattern and clinical outcome of palbociclib in daily practice. Until present, there are no reports of real-world data of palbociclib in Asian countries. Herein authors report the clinical real-world evidence including progression-free survival (PFS), clinical efficacy and toxicities of palbociclib combined with endocrine therapy in Asian real-world practice.
Materials and Methods
1. Patients
From April 2017 to November 2019, the medical records of patients who were diagnosed as HR-positive MBC and received palbociclib were retrospectively reviewed. Patients’ data was collected from eight institutions: Seoul St. Mary’s Hospital (Seoul, Korea), Yeouido St. Mary’s Hospital (Seoul, Korea), Eunpyeong St. Mary’s Hospital (Seoul, Korea), Incheon St. Mary’s Hospital (Incheon, Korea), Uijeongbu St. Mary’s Hospital (Uijeongbu, Korea), Bucheon St. Mary’s Hospital (Bucheon, Korea), St. Vincent’s Hospital (Suwon, Korea), and Daejeon St. Mary’s Hospital (Daejeon, Korea). HR-positive breast cancer was defined as estrogen receptor (ER) or progesterone receptor (PR) positive by American Society of Clinical. Oncology–College of American Pathologists guideline. HER2 negativity was defined as HER2 immunohistochemistry 0/1 (no staining, faint/barely perceptible membrane staining, or weak incomplete membrane staining in < 10% of invasive tumor cells) or HER2 silver in situ hybridization negative (Dual-probe HER2/Chr17 ratio < 2.0 with an average HER2 copy number < 4.0 signals/cell). Luminal A subtype was defined as ER and/or PR positive, HER2 negative with Ki-67 ≤ 20%. Luminal B subtype was defined as ER and/or PR positive, HER2 negative with Ki-67 > 20%. Other eligibility criteria were as follows: (1) receiving at least 1 cycle of palbociclib combined with letrozole or fulvestrant; (2) patients who regularly followed up in eight institutions with adequate medical records.
2. Treatment schedule and response evaluation
Patients were treated with letrozole plus palbociclib or fulvestrant plus palbociclib. Patients received palbociclib 125 mg by oral, 3 weeks on followed by 1 week off schedule (4-week cycle). Letrozole was administered 2.5 mg/day orally daily. Fulvestrant 500 mg was administered intramuscularly on days 1 and 15 of cycle 1 and then every 28 days (±7 days) thereafter starting from day 1 of cycle 1. Premenopausal patients received either bilateral salphingo-oophrectomy (BSO) or gonadotropin-releasing hormone agonist (GnRH agonist) such as goserelin before starting treatment.
Response evaluation was performed based on computed tomography (CT) scans every 3 cycles of treatment, using Response Evaluation Criteria in Solid Tumors (RECIST) criteria ver. 1.1. Toxicity was assessed based on National Cancer Institute Common Terminology Criteria for Adverse Events, ver. 4.0, during each cycle. Treatment was administered until progressive disease or observation of unacceptable toxicity.
3. Statistical analysis
Treatment-free interval (TFI) was defined from the time of completion of adjuvant treatment to cancer recurrence. PFS was calculated from the first start date of letrozole or fulvestrant plus palbociclib administration to the date of disease progression proven by CT scans or patient’s death. Overall response rate (ORR) was defined as patient proportion showing complete response (CR) or partial response over total patient population based on RECIST ver. 1.1. Disease control rate (DCR) was defined as patient proportion with CR, partial response or stable disease over total patient population.
Continuous variables were presented as the median values, and categorical variables were presented as percentages. Continuous variables were compared by Mann-Whitney U test while categorical variables were compared using a chi-square tests and Fisher exact tests. Survival analyses were performed using Kaplan-Meier method and compared with log-rank test. Hazard ratios for PFS were estimated from the Cox proportional hazards model with a 95% confidence interval (CI). Two-sided p-values are presented for all analyses with p < 0.05 considered to be statistically significant. R ver. 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
Results
1. Patient characteristics
Between April 2017 to November 2019, 183 MBC patients treated with palbociclib were enrolled for analysis. Of these patients, 14 patients were excluded for analysis, and total 169 patients were assigned for analysis (Fig. 1). Median follow-up duration was 14.63 months (range, 0.2 to 33.9 months). Baseline patient characteristics are summarized in Table 1. Median age of total patient population was 57 years (range, 37 to 92 years). Most patients showed Eastern Cooperative Oncology Group (ECOG) performance 0 to 1, and 13% patients with ECOG of 2 to 3. Sixty-three patients (37.3%) were diagnosed as de novo stage IV at initial diagnosis. Among stage I to III patients, about 70% of patients received neoadjuvant or adjuvant chemotherapy. Half of patients (55.0%) were presented with visceral disease and 11.8% of patients were diagnosed as bone-only disease. Most patients (97%) were postmenopausal, and 38 patients (22.5%) received BSO for artificial menopause before starting palbociclib.
One hundred and forty-five patients were treated with letrozole plus palbociclib and 24 patients with fulvestrant plus palbociclib. Fulvestrant plus palbociclib treated patients were younger (median age, 53.5 years vs. 58 years) compared to letrozole plus palbociclib group. Fulvestrant plus palbociclib group showed higher disease burden (visceral metastasis, 66.7% vs. 53.1%) with liver metastasis (54.2% vs. 15.2%) compared to letrozole plus palbociclib group.
Letrozole plus palbociclib was mainly administered as first line (93.8%), equal to PALOMA-2 trial. Fulvestrant plus palbociclib group was mostly heavily pretreated, receiving fourth line and beyond (37.5%), including cytotoxic chemotherapy before starting fulvestrant and palbociclib. More than half (58.3%) of patients received cytotoxic chemotherapy before administration of fulvestrant plus palbociclib. Previous treatments administered before palbociclib are described in Table 2.
2. Previous endocrine treatment and pattern of endocrine resistance
Among total patients, 106 patients showed recurrent breast cancer during or after end of adjuvant endocrine treatment (Table 3). Most patients received tamoxifen for adjuvant endocrine treatment. Half of patients did not complete adjuvant endocrine treatment due to cancer recurrence. Endocrine resistance was classified as primary or secondary resistance, based on ABC4 guideline [20]. In letrozole plus palbociclib arm, about one-third of patients showed endocrine resistance, and most patients (85.7%) were presented with secondary endocrine resistance. All patients were endocrine resistant in fulvestrant plus palbociclib arm. Fulvestrant plus palbociclib group showed shorter duration of adjuvant endocrine treatment (median, 4.0 years vs. 4.7 years) with higher portion of endocrine resistance (100% vs. 31.8%) compared to letrozole plus palbociclib group. Median TFI in letrozole plus palbociclib group was 36.03 months.
3. Efficacy
The median progression-free survival (mPFS) of letrozole plus palbociclib was 25.6 months (95% CI, 19.1 months to not reached) (Fig. 2A), comparable to PALOMA-2. The mPFS benefit of luminal A was superior to luminal B patients (hazard ratio, 2.86; 95% CI, 1.20 to 6.80; p=0.017), and good performance status was also associated to improved mPFS (hazard ratio, 3.68; 95% CI, 1.70 to 7.96; p=0.001) (Fig. 3A and B). Interesting finding was that although not statistically significant, elderly patients showed trends for superior mPFS compared to younger patients (Fig. 4A). There were no differences of mPFS according to visceral metastasis or bone-only metastasis at baseline. However, presence of liver metastasis among visceral metastasis was associated to poor survival, reflecting liver metastasis as a poor prognostic factor (Fig. 4A). The ORR and DCR in our study was 39.6% and 89.2%, also comparable to PALOMA-2 (Table 4).

Progression-free survival (PFS) in letrozole (A) or fulvestrant (B) plus palbociclib treated patients. CI, confidence interval; mPFS, median progression-free survival; NA, not available.

Progression-free survival (PFS) of subgroup analysis according to subtype (A), performance status (B) in letrozole plus palbociclib group and previous history of cytotoxic chemotherapy (CTx) (C) in fulvestrant plus palbociclib group. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NA, not available.
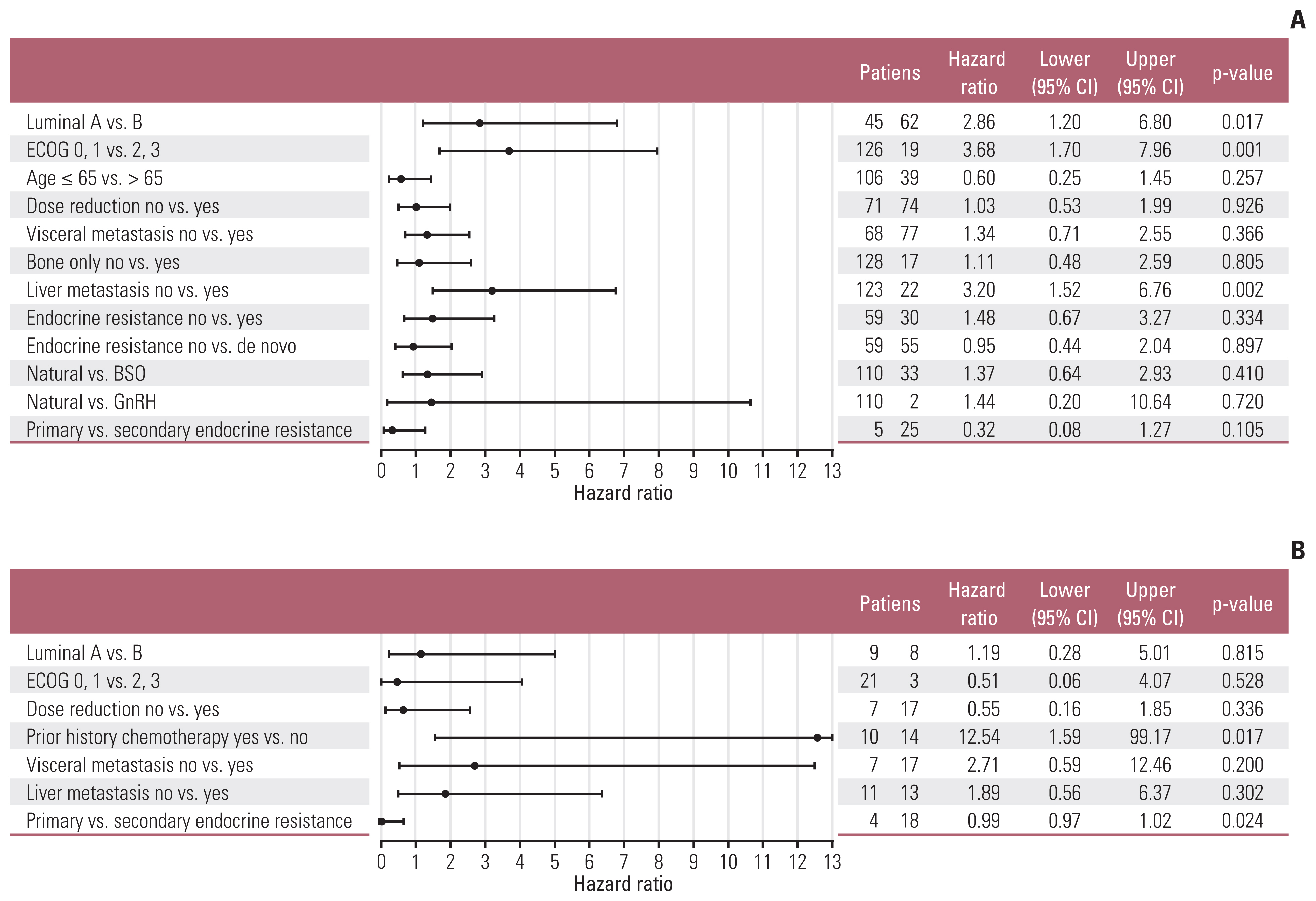
Forest plot of post-hoc subgroup analysis. (A) Letrozole plus palbociclib. (B) Fulvestrant plus palbociclib. BSO, bilateral salphingo-oophrectomy; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; GnRH, gonadotropin-releasing hormone agonist.
mPFS of fulvestrant plus palbociclib was 6.37 months (95% CI, 5.33 months to not reached) (Fig. 1B). In our analysis, nearly half of patients were treated with four or more lines of systemic treatment including endocrine and cytotoxic agent before administration of fulvestrant and palbociclib (Table 2). Patients who were not exposed to cytotoxic chemotherapy before fulvestrant plus palbociclib showed better PFS (mPFS, not reached) compared to patients who were pretreated with cytotoxic chemotherapy (Fig. 3C). There were no mPFS differences according to subtype, presence of visceral metastasis or liver metastasis (Fig. 4B). The ORR and DCR was 28.6% and 81% (Table 4).
4. Benefit of mPFS according to endocrine resistance and methods of ovary function suppression
There was no difference of PFS according to presence of endocrine sensitivity in letrozole plus palbociclib treated patients. No statistical differences were found between de novo stage IV, endocrine sensitive and endocrine resistance patient group in regards of mPFS during analysis (Fig. 5A). However, patients who were classified as primary endocrine resistance showed trends for poor mPFS compared to secondary endocrine resistant patients (Fig. 5B and C).
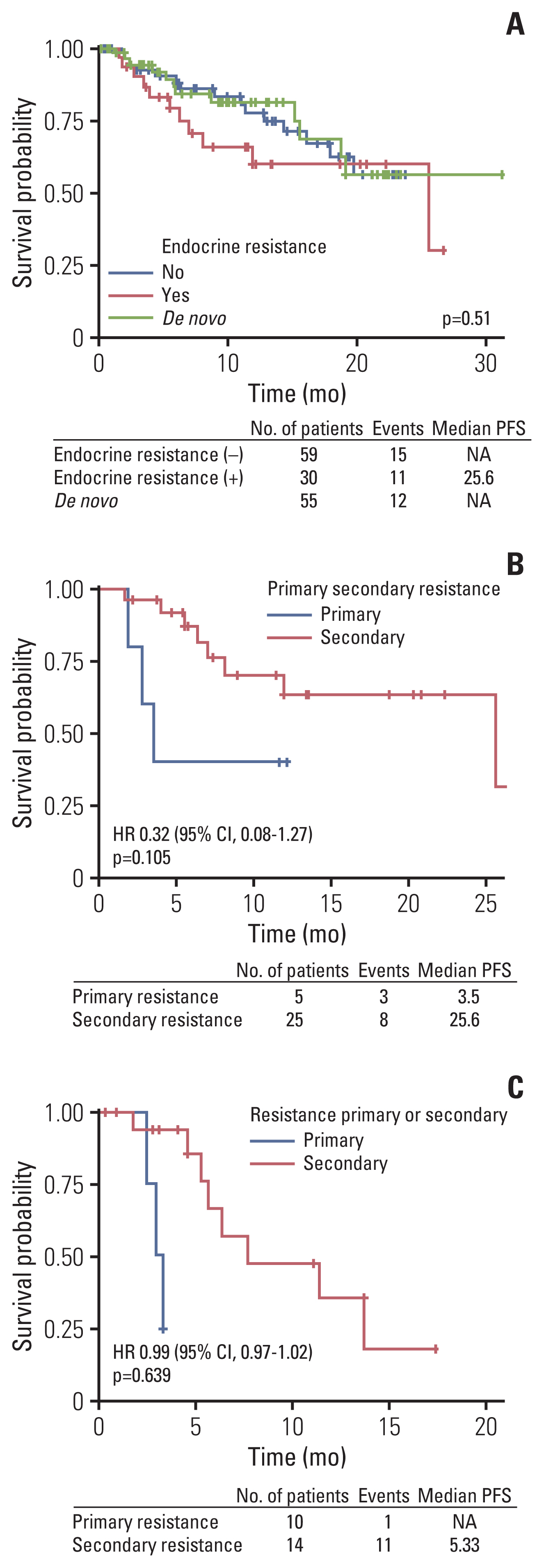
Progression-free survival (PFS) of subgroup analysis according to presence of endocrine resistance (A) in letrozole plus palbociclib group and primary or secondary endocrine resistance in letrozole or fulvestrant plus palbociclib group (B, C). CI, confidence interval; HR, hazard ratio; NA, not available.
In our analysis, most premenopausal patients received BSO for permanent ovary function suppression and became postmenopausal before starting letrozole plus palbociclib as first-line treatment. Very small portion of patients in our study (2 patients, 1.4% among total patients) received GnRH agonist with letrozole and palbociclib. No mPFS difference was observed according to methods of ovary function suppression in letrozole plus palbociclib treated patients (Fig. 4A). This result should be interpreted with caution because small number of patients analyzed, with possibility of selection bias.
5. Concurrent radiation during palbociclib administration
Among total patient population, 28 patients (16.5%) received palliative radiation therapy with concurrent palbociclib administration. Axial skeleton was the most common site for radiation therapy. Chest wall, pelvic bone, extremity bone and breast were treated for palliation in order of frequency. Radiation was delivered with concurrent palbociclib administration, and one-third of patients experienced grade 3–4 neutropenia. There was no febrile neutropenia reported during or after radiation therapy. Half of patients experienced dose delay of palbociclib due to delayed bone marrow recovery or fatigue. One-third of patients received reduced dose of palbociclib after concurrent radiation. Most patients (85.7%) received 21 days of palbociclib administration during concurrent radiation (Table 5). Changes of absolute neutrophil count during and after radiation therapy of representative patients are depicted in supplement figure (S1 Fig.).
6. Safety and dose reduction
The most common adverse events were neutropenia and thrombocytopenia in total patient population (Table 6). The incidence of neutropenia and anemia was similar in both groups, but thrombocytopenia was more frequently detected in fulvestrant plus palbociclib arm. Most common non-hematologic adverse event was fatigue in total patient population.
More than half of patients (74 patients, 51.0%) reduced dose of palbociclib in letrozole plus palbocilib arm. In fulvestrant plus palbociclib group, 17 patients (70.8%) reduced palbociclib dosage. The main cause of dose reduction was delayed bone marrow recovery and fatigue (S2 Table). There was no mPFS difference according dose reduction of palbociclib, irrespective of letrozole or fulvestrant backbone (Fig. 4B).
Discussion
After palbociclib has proven its clinical efficacy in phase 3 trials [10,15], combination of palbociclib with endocrine treatment has become crucial for improving survival in HR-positive MBC patients. The efficacy of palbociclib is reported to be equal irrespective of ethnicity but with different intensity of adverse events [16,17]. In addition, different age population and menopausal status between Asian and Western population should be considered while interpreting the results of PALOMA-2 and 3 [16,17]. Asian patient population and premenopausal women was relatively under-represented in pivotal phase III trials, and the treatment guideline for these patient group was extrapolated from Western and postmenopausal women. There are growing need for clinical trials involving premenopausal patients with diverse ethnic populations, and based on this unmet need, MONALEESA-7 trial enrolled premenopausal women for first-line treatment of ribociclib with aromatase inhibitor and showed promising survival outcome comparable to postmenopausal women. However, still there are unmet needs for Asian and premenopausal patient population and real-world data of CDK 4/6 inhibitors including palbociclib may have certain role in prior patient population.
Real-world data is important for reproducing the efficacy of clinical trials in patient population with heterogenous clinical characteristics. The real-world outcome of palbociclib in Western countries is recently published [21–24], but currently there are no real-world data of palbociclib in Asian countries. Considering Asian patient population and premenopausal women is relatively under-represented in pivotal trials except MONALEESA-7, it is important to analyze the clinical efficacy of palbociclib in real-world to apply it’s use in various clinical situation. To our knowledge, this is the first report about the real-world clinical outcome and treatment patterns of palbociclib in Asian region.
Similar proportion of de novo, endocrine sensitive and endocrine resistant population was enrolled in our study compared to PALOMA-2 trial. At the time point of data analysis, palbociclib was the only approved CDK4/6 inhibitor in Korea. In Korea, HR-positive MBC patients only have a single chance to use CDK4/6 inhibitor including palbociclib during her treatment course. Therefore, nearly all patients used palbociclib as upfront treatment option irrespective of patients’ age, disease burden, and disease-free interval. Due to unique reimbursement issue for palbociclib in Korea, HR-positive breast cancer patients were treated homogeneously with letrozole plus palbociclib as first-line treatment. This unique situation might have affected patient composition in baseline population in our study. The median age of patients treated with letrozole plus palbociclib were 58 years, younger than PALOMA-2. Median age of our study was slightly younger compared to Asian subpopulation data in PALOMA-2. About 25% of patients were premenopausal before starting letrozole plus palbociclib. This proportion or premenopausal women is similar to Korean breast cancer database [25], and most of premenopausal patients received BSO for artificial menopause before starting palbociclib. In addition, most patients with recurrent breast cancer received neoadjuvant or adjuvant chemotherapy compared to PALOMA-2, which may reflect aggressive tumor biology of patients in our study. Despite these unfavorable baseline characteristics, mPFS of patients treated with letrozole plus palbociclib in our analysis was comparable to PALOMA-2.
However, in case of fulvestrant plus palbociclib group, mPFS was inferior compared to PALOMA-3. The median age of patients treated with fulvestrant plus palbociclib was 52.5 years, younger than PALOMA-3. The proportion of premenopausal women before starting fulvestrant plus palbociclib was 37.5%, similar to PALOMA-3. Most premenopausal women received BSO for permanent ovary function suppression. Fulvestrant plus palbociclib treated patients in our analysis were very heavily pretreated with extensive disease burden compared to PALOMA-3. Especially, more than half of patients received at least one line of cytotoxic chemotherapy after recurrence or metastasis. Prior cytotoxic chemotherapy exposure was associated to inferior outcome in fulvestrant plus palbociclib group. These different patient characteristics may have influenced the clinical outcome in our study.
Unlike to Western, incidence of recurrent breast cancer or MBC in premenopausal patients is higher in Asian countries [6]. Although there are significant portion of HR-positive breast cancer patient in premenopausal population, only postmenopausal patients were enrolled in clinical trials and the efficacy of CDK4/6 inhibitors including palbociclib in premenopausal women was extrapolated from the data of postmenopausal patients. In our study, quarter of patients were premenopausal at the time of cancer recurrence or initial diagnosis and showed similar survival outcomes regardless of menopausal status at the time point of cancer recurrence or diagnosis. Before approval of palbociclib, premenopausal women tended to receive cytotoxic chemotherapy rather than endocrine treatment with ovary function suppression in Korea [26,27]. This non-adherence of guideline to administer endocrine treatment unless patient present with visceral crisis was partly due to aggressive biologic behavior of premenopausal women. Other than biologic behavior, lack of approved endocrine treatment with medical ovary function suppression in premenopausal women mostly influenced increased rate of cytotoxic chemotherapy in Korean women. Before national reimbursement of aromatase inhibitor with GnRH agonist since May 2017, premenopausal women had no other option than cytotoxic chemotherapy for systemic treatment. Palbociclib was approved at August 2016, and started nationwide reimbursement since November 2017. After initiation of national insurance program from November 2017, there was a nationwide increase in the number of BSO cases across Korea. Most premenopausal patients received BSO for ovary ablation, due to limited options of ovary function suppression in Korea. In case of eight institutions participating in this study, 59 premenopausal breast cancer women received BSO during recent 2-year period (January 2017–November 2019). Considering there was 111 BSO cases during previous 8 years (January 2009–December 2016), we can assume that there was rapid increase of BSO after approval of palbociclib (S3 Fig.). Although data was selected from eight medical centers in Korea, each center act as major tertiary referral centers and may act as sample group reflecting treatment pattern in Korean patients.
Compared to surgical ovary ablation, very small portion of patients received GnRH agonist for medical ovary function ablation. Among letrozole plus palbociclib treated patients, there were no difference of mPFS according to menopausal status nor the method of ovary function suppression. Although small number of patients were analyzed, this result can be one of an evidence that cautiously suggest palbociclib plus endocrine treatment may benefit in premenopausal patients, and adequate medical ovary function suppression with GnRH agonist may have similar outcome compared to menopausal patients. This finding is in concordance to MONALEESA-7 trial [18], and our data humbly suggest premenopausal patients can be treated equally to postmenopausal patients when adequate ovary function suppression is based. Furthermore, authors presumed with caution that medical ovary function suppression can substitute surgical ovary ablation, but further studies are warranted for confirmation due to the limited evidence.
As reported in PALOMA-2 and 3, palbociclib plus letrozole or fulvestrant showed mPFS benefit irrespective of previous exposure to endocrine treatment. However, primary endocrine resistant patients showed trends for inferior clinical efficacy compared to secondary endocrine resistant patients. CDK4/6 inhibitors showed clinical benefit irrespective of prior endocrine treatment in phase 3 trials, but there are limited data of treatment outcome in primary endocrine resistant subgroup at present. In our analysis, primary endocrine resistant patients showed inferior mPFS compared to secondary resistant patients. Based on this observation, authors surmised that adding palbociclib to endocrine therapy could not reverse the clinical outcome of primary endocrine resistance. CDK4/6 inhibitors are gaining its evidence as upfront treatment options even if patients present with endocrine resistance or visceral crisis [28]. In MONARCH-2, fulvestrant plus abemaciclib showed survival benefit in primary endocrine resistant patients, compared to fulvestrant monotherapy [13]. Still, the outcome of primary endocrine resistant breast cancer may be inferior compared to secondary endocrine resistant population.
In our study, palliative radiation with concurrent palbociclib administration was shown to be relatively feasible with tolerable toxicity profile. There are preclinical data that palbociclib may enhance the effect of radiation when administered concurrently [29]. HR-positive MBC patients frequently present with symptomatic bone metastasis requiring palliative radiation therapy. However, considering the main adverse event of palbociclib is neutropenia, many physicians are reluctant to use palbociclib concurrently with radiation. Previous study reported 16 patients receiving palbociclib with radiation therapy, with tolerable toxicity profile [30]. However, previous study administered palbociclib in close time of period with radiation therapy. We presented 28 patients who were concurrently treated with palliative radiation therapy with palbociclib, and this is the largest study reporting the safety of simultaneous administration of palbociclib with radiation therapy.
Luminal A subtype showed superior mPFS compared to luminal B in letrozole plus palbociclib group. Recent biomarker analysis reported that addition of palbociclib has proven similar survival benefit in luminal A and B subtype [31]. Survival difference between luminal A, B subtype in our analysis may reflect the distinct biologic behavior according to luminal subtype of breast cancer, aligned with PALOMA-2 data [31].
Although not statistically significant, elderly patients showed trends for superior mPFS compared to younger patients in current analysis. This result is in concordance with recent meta-analysis reporting elderly patients who were treated with CDK4/6 inhibitor with aromatase inhibitor presenting superior survival with comparable toxicity to younger patients [32]. Considering the small patient population analyzed, this result needs to be interpreted with caution. Nevertheless, prescribing CDK4/6 inhibitor including palbociclib in elderly population can be considered with care if patient is within medically fit condition.
Palbociclib combination benefited in patients irrespective of visceral metastasis. Patients with visceral metastasis showed similar clinical benefit compared to non-visceral disease group, reflecting the clinical benefit of palbociclib in total patient population. In letrozole plus palbociclib group, patients with liver metastasis showed worse survival outcome. This result is in concordance to subgroup analysis of PALOMA-2 and 3 [33], reflecting presence of liver metastasis as poor prognostic marker in HR-positive MBC patients. There was no survival benefit according to bone-only disease in letrozole plus palbociclib treated patients. However, in fulvestrant plus palbociclib treated group, bone-only disease patients showed trends for superior survival, but this result should be interpreted with caution due to small number of patient population.
Incidence of neutropenia was higher than Western real-world data [21], and similar compared to Asian data of PALOMA-2 and 3 [16,17]. Higher rate of adverse events resulted in more frequent dose reduction, compared to phase 3 trials. More than half of patients in letrozole plus palbococlib arm experienced dose reduction, and 76% of patients in fulvestrant plus palbociclib group reduced palbociclib dosage due to any adverse events. Dose reduction rate in letrozole plus palbociclib group in our study was similar to Asian subgroup analysis of PALOMA-2 [16]. Fulvestrant plus palbociclib treated patients in our analysis experienced higher dose reduction rate compared to Asian subpopulation in PALOMA-3 [17]. In the study, fulvestrant plus palbociclib group were more heavily pretreated, and this previous treatment may have affected dose reduction rate of palbociclib. There was no mPFS difference according to presence of dose reduction, and this is in concordance to Asian data of PALOMA-2 and 3 [16,17].
There are some limitations to mention in this study. Events such as progression or cancer-associated death was relatively limited due to short follow-up duration and some follow-up loss data may have influenced the favorable mPFS data in our analysis. Longer follow-up of our data is warranted for confirmation of mPFS in patients treated with palbociclib in our study. Furthermore, although authors have intensively reviewed the medical records of enrolled patients, there were some missing data such as laboratory findings and incomplete assessments of toxicities. Nevertheless, our study has its strength as one of an Asian real-world data of palbociclib used in HR-positive MBC in real-world setting. We have demonstrated the clinical benefit of palbociclib in Asian population is similar to the phase 3 data, and feasible to use in patients in real-world. Sufficient long-term follow-up is needed to analysis the role of palbociclib affecting overall survival of Asian patients in real-world setting.
In conclusion, palbociclib plus letrozole or fulvestrant was well tolerated in Asian patients with concordant PFS compared to phase 3 trials. Combination of palbociclib to letrozole or fulvestrant with sufficient ovary function suppression such as GnRH agonist or BSO showed comparable clinical benefit to postmenopausal patients. The incidence of adverse events and dose reduction was higher compared to phase 3 trials as expected, but similar to subgroup Asian data of PALOMA-2 and 3. Administration of palbociclib was feasible in real-world Asian population, with manageable toxicity profiles.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Catholic Medical Center, The Catholic University of Korea (XC19REDI0057). The requirement for written informed consent was waived according to the decision of IRB.
Author Contributions
Conceived and designed the analysis: Lee J, Chun SH, Byun JH.
Collected the data: Lee J, Park HS, Won HS, Yang JH, Lee HY, Woo IS, Shin K, Hong JH, Yang JY, Chun SH, Byun JH.
Contributed data or analysis tools: Lee J, Park HS, Won HS, Yang JH, Lee HY, Woo IS, Shin K, Hong JH, Yang YJ, Chun SH, Byun JH.
Performed the analysis: Lee J.
Wrote the paper: Lee J.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
We thank Dr. Hyun Seon Kim for providing the data for the manuscript.






