Radiation-induced Necrosis Deteriorating Neurological Symptoms and Mimicking Progression of Brain Metastasis after Stereotactic-guided Radiotherapy
Article information
Abstract
Purpose
Although radiation-induced necrosis (RIN) is not a tumor in itself, the lesion progressively enlarges with mass effects and diffuse peritumoral edema in a way that resembles neoplasm. To identify the RIN that mimics progression of brain metastasis, we performed surgical resections of symptomatic RIN lesions.
Meterials and Methods
From June 2003 to December 2005, 7 patients received stereotactic-guided radiotherapy (SRT) for metastatic brain tumor, and they later underwent craniotomy and tumor resection due to the progressive mass effects and the peritumoral edema that caused focal neurological deficit. On MR imaging, a ring-like enhanced single lesion with massive peritumoral edema could not be distinguished from progression of brain metastasis.
Results
Four patients had non-small cell lung cancer, 2 patients had colorectal cancer and 1 patient had renal cell carcinoma. The mean tumor volume was 8.7 ml (range: 3.0~20.7 ml). The prescribed dose of SRT was 30 Gy with 4 fractions for one patient, 18 Gy for two patients and 20 Gy for the other four patients. The four patients who received SRT with a dose of 20 Gy had RIN with or without microscopic residual tumor cells.
Conclusions
Early detection of recurrent disease after radiotherapy and identifying radiation-induced tissue damage are important for delivering adequate treatment. Therefore, specific diagnostic tools that can distinguish RIN from progression of metastatic brain tumor need to be developed.
INTRODUCTION
Stereotactic-guided radiotherapy (SRT) is being increasingly used for patients suffering with intracranial metastatic tumors. SRT was first introduced in 1949 (1) but it was not used to treat brain metastases (BM) until the 1980's (2). Theoretically, BM are ideal targets for SRT (3). The vast majority of these lesions are round or pseudospherical (4), and it is not difficult to achieve a spherical isodose configuration when planning SRT treatment (5). BM are often located in noneloquent areas at the gray-white matter junction (4), allowing the delivery of a single large fraction dose with relatively low morbidity (3,6~9). Although the patients treated with SRT may have low morbidity, several patients have suffered from radiation induced necrosis (RIN) that caused focal neurological deficit. These conditions appear within the irradiated volume as contrast-enhancing, expansive brain lesions surrounded by edema. Traditionally, brain toxicity after radiation therapy (RT) has been considered to have an association with treatment related necrosis (10). Single-dose equivalent mathematical models can reliably predict the 1% and 3% risks of RIN, based on the radiation dose and the treated brain volume, respectively (11).
It is important to differentiate RIN from progression of BM or residual tumor for delivering the proper treatment to patients. Yet making the differential diagnosis between tumor recurrence and RIN is difficult after radiotherapy for brain tumors with using the conventional neuro-imaging modalities (12). Although several diagnostic tools have been developed, including [2-18F] fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET), methionine PET and proton MR spectroscopy (1H-MRS), it is still difficult to differentiate progressive or recurrent BM from radiation injury after RT.
In this study, we performed surgical resection of metastatic brain tumors that were considered to be progression of brain tumor after SRT, and we compared the actual pathological findings.
MATERIALS AND METHODS
Forty-five patients with metastatic brain tumor were treated with SRT in a single fraction or several fractions at our institute from June 2003 to December 2005. Among these patients, 7 patients later underwent craniotomy and tumor resection due to the progressive mass effect that caused focal neurological deficit.
Radiation-induced necrosis was defined as a form of coagulation necrosis combined with fibrinoid necrosis of blood vessels and hyalinization of the vascular walls within the previous radiation field and the gadolinium (Gd)-enhanced area on the T1-weighted MR imaging.
The surgical indications included the signs and symptoms of intracranial hypertension that was unresponsive to adequate medical therapy such as corticosteroid and mannitol, intractable seizures, a decreased level of consciousness, progressive motor weakness and speech disturbance. On the neuro-imaging studies, enlarging lesion, hemorrhage and a mass effect from edema that was unresponsive to maximal medical therapy were also considered for surgical resection.
The medical records of all the patients were analyzed, including the clinical history, the operative and pathology reports and the radiologic studies, and the dates of death were confirmed for all the patients who died.
SRT was tried with a single fraction in 6 patients and fractionated SRT (FSRT) by four fractions was done in 1 patient; both techniques were performed with using a 6-MV beam (CL600CD; Varian Medical Systems, Palo Alto, CA) equipped with a leaf width of 3 mm (m3; BrainLAB, Heimstetten, Germany). The total dose ranged 18 to 20 Gy in a single fraction, and 30 Gy in four fractions, which were prescribed at 95% (range: 90~97%) and 85% of the isodose surface of the maximum dose, respectively. The dose rate was 300 cGy per minute. Targeted and critical structures such as the optic nerves, brain stem, eyes and optic chiasm were identified and outlined on the pretreatment MR imaging (MRI), as was visualized on the treatment planning software (BrainSCAN, Heimstetten, Germany). The CT images were acquired using a 2-mm slice thickness. The CT and MRI scans were fused, and a stereotactic conformal plan was created for the target by using multiple noncoplanar fixed beams. Single isocenter treatment plans were accomplished in all patients. The patients were treated with five to eleven fixed beams.
RESULTS
1) Characteristics of the patient population
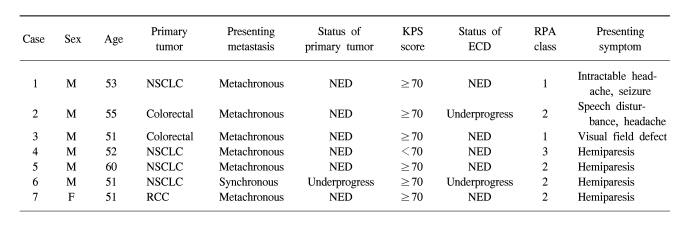
A summary of patient characteristics is shown in Table 1. There were 7 patients with a mean age of 53.3 years (range: 51~60 years) at the time of undergoing SRT. Four patients had non-small cell lung cancer, 2 patients had colorectal cancer and 1 patient had renal cell carcinoma (RCC). Six patients had metachronous presentations with the initial diagnosis of primary disease. Two patients had extracranial metastases. Six patients had successful primary tumor control at the time of performing SRT. Six patients had Karnofsky Performance Scale (KPS) scores of 70 or more. For the Recursive Partitioning Analyses (RPA) class, two patients were grade 1, another four patients were grade 2 and the other one patient was grade 3. Four patients suffered mainly from hemiparesis, one from intractable headache, one from speech disturbance and the other one from visual field defect. Six patients received previous systemic chemotherapy and three patients underwent systemic chemotherapy following SRT.
2) Features of brain lesions
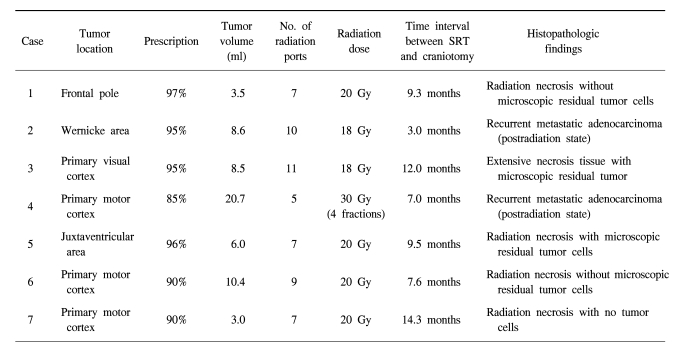
Three tumors were located in the primary motor cortex, one in the primary speech center, one in the primary visual cortex, one in the deep white matter (the juxtaventricular area) and the other one was in the left frontal pole. Patient 1 refused craniotomy for metastatectomy and wanted to undergo SRT. The mean tumor volume was 8.7 ml and this ranged from 3.5 to 20.7 ml. The mean largest diameter of tumor on the T1-weighted MRI with Gd enhancement was 20.6 mm and this ranged from 15 to 27.5 mm. The features of metastatic brain lesions are shown in Table 2.
3) Clinical course and treatments
All the patients were followed up for 10 months or more and the mean follow up duration was 17.2 months; this ranged from 10 to 29.2 months.
For each patient, during the follow-up period after SRT, MRI showed the progressive space-occupying lesion as a ring-like enhanced mass with extensive peritumoral edema and mass effects. The peritumoral edema was related to progression of the neurological deficit. All the patients were treated with conservative care using steroid and mannitol for 1 week or less, and they experienced temporary improvement, but the neurological deterioration returned and accelerated. Therefore, these patients underwent surgical resection to reduce the mass effect and improve their neurological deficits. The mean time interval between SRT and the later craniotomy was 9.0 months and this ranged from 3.0 to 14.3 months. Among the four patients who had hemiparesis before craniotomy, three patients experienced improvement of their motor weakness, and the other three patients who had focal neurological deficit and intractable headache during follow-up after SRT experienced a great deal of improvement.
Three patients died during follow-up due to primary disease progression and the survival times were 10.0, 11.2 and 15.4 months, respectively.
4) Histopathological results
For the patient who received FSRT with dose of 30 Gy, progressive, space occupying, ring-like enhanced lesion was found as recurrent metastatic adenocarcinoma in the tissue obtained by mass resection. In the patients who received SRT with a single dose of 18 Gy, one of two brain lesions was recurrent metastatic adenocarcinoma with mixed necrotic tissue, and another was extensive necrosis tissue with microscopic residual tumor. In the other four patients who received SRT with a single dose of 20 Gy, all of them had RIN with or without microscopic residual tumor cells. Table 2 shows the relationship between the SRT dose and the histopathological findings.
5) Illustration of case
A 51-year-old man had a symptomatic mass lesion in the right precentral gyrus, as shown by follow-up MRI. The lesion was diagnosed as a metastatic brain tumor by Gd-enhanced T1-weighted MRI. He had non-small cell lung cancer and had been treated with systemic chemotherapy (combined gemcitabine and cisplatin) 2 months previously. Therefore, he underwent SRT with a single dose of 20 Gy for the brain metastasis that was causing left hemiparesis. After SRT, his motor weakness was immediately improved. He then continued to receive systemic chemotherapy for his primary lung cancer. However, 8 months later, he developed difficulty in walking due to left hemiparesis, and a ring-like enhanced lesion with peritumoral edema was observed in the right precentral gyrus on MRI.
His walking improved temporarily in response to 1 week of conservative treatment with steroid and mannitol, but thereafter he progressively deteriorated despite continuing the conservative therapy. Therefore, total removal of the progressive space occupying, ring-like lesion was performed at 2 weeks after the deterioration of the patient's ability to walk. The histopathological diagnosis was RIN with no tumor. After surgery, the peritumoral edema seen on MRI and the gait disturbance were improved. The MR imaging and histopathological findings are showed in Fig. 1 and 2.
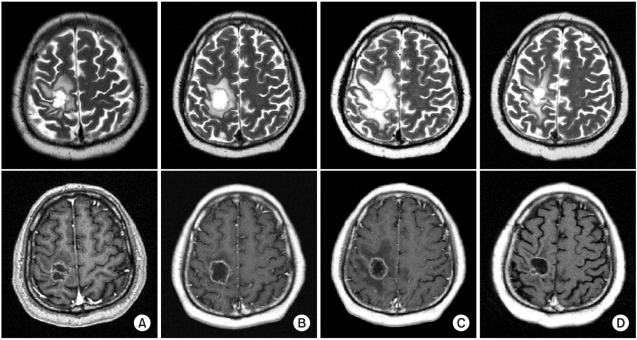
MR imaging findings. At diagnosis, MRI shows a ring-like enhanced mass in the right precentral gyrus in T1-weighted image with Gadolinium enhancement and combined peritumoral edema in T2-weighted image (A). One month after SRS, increased central necrosis and still remained peritumoral edema were shown (B). Eight months after SRS, progressive increased extent of peritumoral edema without significant change in ring-like enhancing lesion (C). After surgical resection of brain lesion, surgical defect was found at the previous tumor site and decreased signal intensity of peritumoral edema (D).

Histopathological findings in tissue from the zone of Gd-enhancement on MRI shows characteristics of radiation necrosis. There is coagulative type necrosis of the normal brain. The blood vessels show fibrinoid necrosis and hyalinization of the vascular walls. In the left lower of this figure, minimal cytologic atypia or reactive glial cells is noted (H&E staining, ×100).
DISCUSSION
In this study, we performed surgical resection of brain metastasis that caused deteriorating neurological symptoms after SRT and we analyzed the pathological results. Therefore, in some cases, the RIN was found to mimic progression of brain disease, and in some cases, progression of BM was confirmed by pathologic examinations.
The early detection of recurrent disease after RT and the identification of radiation-induced tissue damage are important for delivering adequate treatment. Using conventional CT, MRI and FDG-PET, it is difficult to differentiate between the lesions related to residual or recurrent brain tumor and the lesions related to non-tumorous, post-irradiation reaction. In several reports, the authors state that FDG-PET scanning is a useful technique for examining either tumor recurrence or RIN (13). Although FDG-PET scanning is generally an excellent procedure for differentiating recurrent tumor from the residual tumor caused by radiation injury, making the differential diagnosis can be difficult for some cases of recurrent BM with FDG hypometabolism. Kline et al showed that the sensitivity and specificity of 201ThalIium(TI)-SPECT scanning for distinguishing RIN from brain tumor recurrence were 92% and 67%, respectively, (14). But increased 210TI uptake has been observed in both RIN and inflammatory infectious processes; this procedure has a limitation for distinguishing RIN from non-neoplastic processes.
Unfortunately, in our study, we did not perform other imaging studies such as FDG-PET or MR spectroscopy to distinguish RIN from the progression of BM because the patients were experiencing urgent neurological deterioration. In fact, if patients suffer from rapid neurological decline, then they should be considered for undergoing surgical decompression for the brain lesion that causes a mass effect or for treating extreme peritumoral edema, and this could be done even without differentiating RIN from BM progression.
Several investigators have described the advantages of SRT as rapid symptomatic improvement, excellent local control, its applicability for deep seated tumors that are difficult to reach surgically, the low morbidity, the shorter hospital stay and reduced costs (15~17). The goal of SRT is to control disease with fewer complications than those complications resulting from alternative treatments. However, using high radiation doses still bear an increased risk of focal radiation injury to the brain because the irradiated target volume includes a safety margin of healthy tissue around the gross tumor volume. In patients who received an overdose of irradiation, it was thought that the normal white matter might also be severely damaged, so that the lesion might be resistant to conservative treatment. In fact, if the RIN after SRT has an association with mass effects and severe peritumoral edema, then such patients can not avoid undergoing surgical resections.
Surgical extirpation and steroid therapy are valuable treatments for progressive cerebral RIN with a mass effect and massive peripheral edema, and the prognosis of patients who underwent surgical extirpation seems to be slightly better than those that undergo steroid therapy alone (18). But removal of progressive RIN should be performed at an early stage before the lesion becomes irreversible.
RIN occurs as the result of the late delayed effects of RT and it usually develops several months or years after RT (19). The incidence of RIN has been reported to range from 1% to 15% after brain irradiation (18). Damage of the endothelial cells by radiation leads to an increase in vascular permeability (20), which produces perivascular edema (21) and demyelinization phenomena. Vascular collapse in the white matter may then interfere with the cerebral blood flow and energy supply to the tissue (and particularly the white matter), leading to brittle parenchyma and promoting the appearance of disorganized and/or destroyed brain tissue due to the break down products of myelin and the response of the microglial cells. This mechanism generates a vicious cycle and worsening pathological change (22). Therefore, the aim of surgical resection is to break the vicious cycle in progressive RIN.
Several factors have been examined for their correlation with the local control achieved by SRT. Akio et al suggested that tumor size had an impact on the radiographic response to SRT (23). Schomas et al reported that the prescribed dose, tumor volume and median minimum target doses have significant relation with local control (24). They suggested that excellent local control rates were seen for those lesions treated with ≥14 Gy. Hoffman et al reported local control rates of 28%, 82% and 95% with doses of <15 Gy, 15~17.9 Gy, and ≥18 Gy, respectively, (p=0.008) (25). In this study, four patients received SRT with a dose of 20 Gy, and all of them had RIN with or without microscopic residual tumor cells. Yet the tumor volume was not associated with the occurrence of RIN in the present study.
CONCLUSIONS
Radiation-induced cerebral necrosis is not a neoplasm, but the lesion tends to progressively enlarge with a mass effect and diffuse peritumoral edema in a way that resembles neoplasm. Thus, those patients who undergo SRT with an excessive dose suffer from neurological symptoms. In the case of patients who are refractory to the conservative treatment with steroid and mannitol, they must undergo surgical removal of RIN. Although it is important to detect radiation injury early for the proper care of patients, there is still no definitive and conformal diagnostic tool to differentiate RIN from progression of BM.