Retrospective Molecular Epidemiology Study of PD-L1 Expression in Patients with EGFR-Mutant Non-small Cell Lung Cancer
Article information
Abstract
Purpose
Data are limited on programmed death ligand 1 (PD-L1) expression in epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC).
Materials and Methods
We retrospectively evaluated the relationship between PD-L1 expression and recurrence-free survival (RFS) and overall survival in 319 patients with EGFR-mutant NSCLC who were treated at Samsung Medical Center from 2006 to 2014. Membranous PD-L1 expression on tumor cells was measured using the PD-L1 IHC 22C3 pharmDx antibody and reported as tumor proportion score (TPS). Kaplan-Meier methods, log-rank test, and Cox proportional hazards models were used for survival analysis.
Results
All patients had ≥1 EGFR mutation—54% in exon 19 and 39% in exon 21. Overall, 51% of patients had PD-L1–positive tumors. The prevalence of PD-L1 positivity was higher among patients with stages II-IV versus stage I disease (64% vs. 44%) and among patients with other EGFR mutations (75%) than with L858R mutation (39%) or exon 19 deletion (52%). PD-L1 positivity was associated with shorter RFS, with an adjusted hazard ratio of 1.52 (95% confidence interval [CI], 0.81 to 2.84; median, 18 months) for the PD-L1 TPS ≥ 50% group, 1.51 (95% CI, 1.02 to 2.21; median, 31 months) for the PD-L1 TPS 1%-49% group, and 1.51 (95% CI, 1.05 to 2.18) for the combined PD-L1–positive groups (TPS ≥ 1%) compared with the PD-L1–negative group (median, 35 months).
Conclusion
PD-L1 expression is associated with disease stage and type of EGFR mutation. PD-L1 positivity might be associated with worse RFS among patients with surgically treated EGFR-mutant NSCLC.
Introduction
Approximately 80%-85% of all lung cancers worldwide are non-small cell lung cancers (NSCLCs) [1]. There are three main subtypes of NSCLC, of which adenocarcinoma is the most frequent histologic subtype [2]. Though histologic subtype provides a basis for choice of chemotherapy, overall survival (OS) remained poor regardless of subtype of NSCLC [3,4].
The emergence of targeted therapy for driver oncogenes involved in NSCLC revolutionized its treatment [3]. Early therapies targeted epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations [3,4]. Patients without these oncogenes receive platinum-based chemotherapy as first-line therapy, whereas patients with activating EGFR mutations receive tyrosine kinase inhibitors (TKIs) as first-line therapy [1,3,4]. Among Asian patients with NSCLC, 30%-51% have EGFR mutations [2,5] compared with < 10% of non-Asian patients, which can improve the survival of Asian patients with advanced or recurrent NSCLC [2]. However, most patients who respond to these therapies eventually develop resistance and progress.
Some tumors, including NSCLC, have been shown to avoid destruction by the innate immune system by overexpression of programmed death 1 (PD-1) ligands [6-8]. Our previous results suggested that positive PD ligand 1 (PD-L1) expression may be a negative prognostic factor among patients with NSCLC who underwent surgery, most notably among patients with adenocarcinoma [9,10]. Thus, targets of the PD-1 pathway are an appealing option for potential therapeutics. Two humanized anti–PD-1 antibodies (nivolumab and pembrolizumab) [11,12] and 1 humanized anti–PD-L1 antibody (atezolizumab) [13] are currently approved in the United States for treatment of NSCLC, and several additional monoclonal antibodies targeting the PD-1/PD-L1 pathway are under investigation [10,14-17].
To date, there are limited data on PD-L1 expression in patients with EGFR-mutant NSCLC and whether the prevalence or the prognostic effect of PD-L1 expression may vary among different types of EGFR mutations. We evaluated the relationship between PD-L1 expression and clinical characteristics, including age, sex, smoking history, stage, and recurrence-free survival (RFS), and OS, among patients with surgically resected EGFR-mutant NSCLC, using the U.S. Food and Drug Administration (FDA)–approved companion diagnostic immunohistochemical assay PD-L1 IHC 22C3 pharmDx (Dako North America, Carpinteria, CA) [18,19].
Materials and Methods
1. Study design and patients
A total of 5,505 patients with NSCLC (2,815 of these patients with adenocarcinoma) underwent surgical resection at Samsung Medical Center (Seoul, Korea) between April 1, 2006, and January 31, 2014. Eligible patients in this study were those who had histologically confirmed NSCLC, confirmed EGFR mutation, sufficient tissue for PD-L1 immunohistochemical staining, and complete clinical and outcome information (n=323). EGFR mutation status was confirmed using direct sequencing or peptide nucleic acid–clamp method of exon 18 through 21 of chromosome 7 at Samsung Medical Center. Baseline demographics and disease characteristics (including age, sex, histologic type, stage, and Eastern Cooperative Oncology Group performance status) and clinical outcomes (including treatment and dates of diagnosis, surgery, and death or recurrence) were retrospectively obtained from medical records. Pathologic tumor stage was defined using the American Joint Committee on Cancer cancer staging manual, seventh edition [20]. Stage was assigned retrospectively for patients whose tumors were staged before publication of the seventh edition. Smoking status was defined as never (< 100 lifetime cigarettes) or current (quit < 1 year before diagnosis). The study was approved by the appropriate institutional review board.
2. PD-L1 immunohistochemistry
PD-L1 expression was assessed in formalin-fixed paraffin-embedded tumor samples acquired by surgical resection of each patient’s individual sample, using the PD-L1 IHC 22C3 pharmDx assay (Dako North America). All PD-L1 assays were performed at LabCorp Clinical Trials, Los Angeles (Laboratory Corporation of America, Burlington, NC), the same laboratory, using the same procedures, that was employed in the pembrolizumab clinical trials for NSCLC [21,22]. The assay is described in detail elsewhere [18,19]. Membranous PD-L1 expression on tumor cells was defined by tumor proportion scores (TPSs) of ≥ 50% and 1%-49%, respectively (Fig. 1), consistent with the cutoffs used in pembrolizumab clinical trials [21,22].
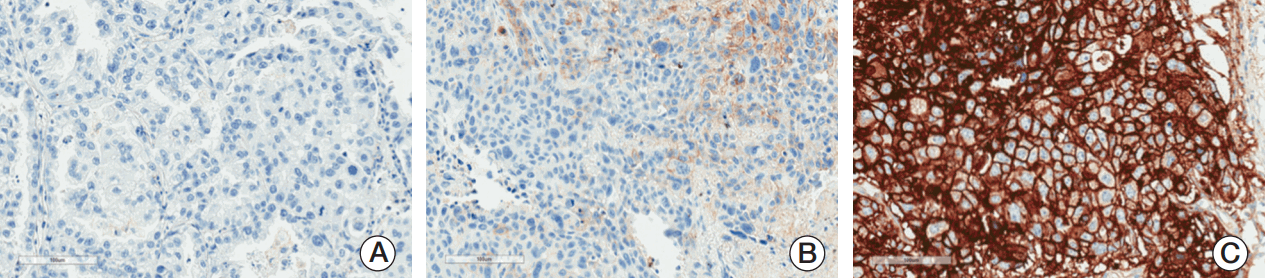
Sample images of programmed death ligand 1 (PD-L1) staining in non-small cell lung cancer. (A) PD-L1 negative (tumor proportion score [TPS] < 1%). (B) PD-L1 TPS 1%-49%. (C) PD-L1 TPS ≥ 50%. All images are at original magnification ×20, blue counterstain is hematoxylin, and PD-L1 is identified by brown chromagen.
3. Statistical methods
The prevalence of PD-L1 TPS ≥ 50% and TPS 1%-49% was compared with the use of chi-square analysis in different subgroups based on age, sex, smoking status, stage, and types of EGFR mutations. RFS was defined as time from the date of diagnosis to the date of recurrence, death, or last follow-up. OS was defined as time from the date of diagnosis to the date of death or last follow-up. Kaplan-Meier method, log-rank test, and Cox proportional hazards models were used to analyze the relationship between PD-L1 expression and RFS and OS, with the PD-L1–negative group as the reference group.
Two adjusted Cox proportional hazards models were used. In each model, the PD-L1–negative population was the reference population. The first Cox model adjusted for known baseline prognostic factors, including age, sex, smoking status, disease stage, and Eastern Cooperative Oncology Group performance status. The second Cox model adjusted for these known baseline prognostic factors, as well as postsurgical chemotherapy, including EGFR TKI and radiation therapy. Different types of EGFR mutations were also included in the initial models, and covariates included in the final models were based on a backward stepwise variable selection process. Statistical analyses were performed using SAS ver. 9.3 (SAS Institute Inc., Cary, NC); p ≤ 0.05 was considered to be statistically significant.
Results
1. Patient population
Among the 323 patients who were included in the original data set, PD-L1 expression status for four patients could not be evaluated because of insufficient tumor cells in the slides, leaving 319 patients in the final analysis. Among those, median age was 62.0 years (range, 35 to 84 years), 61% were women (n=194), 39% were men (n=125), 64% were never smokers (n=205), and 36% were smokers (n=114). The majority of the patients (n=311, 97%) had adenocarcinoma, reflecting the clinical representation of EGFR mutations in Asian populations. Most patients had early-stage disease, including 49% (n=153) stage IA, 15% (n=48) stage IB, 13% (n=41) stage II, and 20% (n=61) stage III. Additionally, 5% (n=16) were stage IV.
2. Treatment information
All 319 patients received surgery, with 94% of curative intent. Additionally, 30% (n=95) received postsurgery adjuvant chemotherapy including five patients had EGFR TKIs (mainly gefitinib for the palliative purpose due to pleural seeding during the surgery), and 10% (n=33) received adjuvant radiation. A total of 128 patients had recurrence, among whom 84% (n=108) received postrecurrence EGFR TKIs, and 59 of the 108 patients also received additional chemotherapy; 39% of the 128 patients (n=50) received postrecurrence radiation.
3. EGFR, KRAS, and ALKstatus
All 319 patients had tumors with at least one EGFR mutation (Table 1). Among the EGFR mutations, 171 patients (54%) had a mutation in exon 19, including 145 with a deletion and others with complex mutations; 124 patients (39%) had a mutation in exon 21, including 121 with an L858R mutation and three with other mutations; 12 patients (4%) had a missense mutation in exon 18; and eight patients (2%) had a mutation in exon 20 including insertion, deletion, and missense mutations. One patient had mutations in both exons 18 and 20, and three patients (1%) had other mutations.
Among the 319 patients, 305 were also tested for Kirsten rat sarcoma (KRAS) mutation at exons 12, 13, and 61. Only one patient was confirmed as KRAS mutation positive at exon 13 with PD-L1 TPS of 1%-49%. Two-hundred eighty patients had ALK status by immunohistochemistry; only two were ALK positive with both PD-L1 TPS of 1%-49%. Three patients were checked for ALK gene rearrangement status by fluorescence in situ hybridization, and all were ALKnegative.
4. PD-L1 status
A total of 163 patients (51%) had PD-L1–positive tumors, including 24 (8%) with TPS ≥ 50% and 139 (44%) with TPS of 1%-49%. Similar to previous results [9], higher rates of PD-L1 positivity were observed among men (vs. females; chi-square p=0.008), smokers (vs. never smokers; chi-square p=0.011), and patients with more advanced disease (stages II/III/IV vs. I; chi-square p=0.006) (Table 1). PD-L1 positivity was less common in patients with exon 19 deletion (76 of 145, 52%) or exon 21 L858R mutation (47 of 121, 39%) compared with other EGFR mutations (40 of 53; 75%; chi-square p=0.001) (Table 1), which include missense mutations in exon 18 (n=12), insertion, deletion, and missense mutations exon 20 (n=8), other mutations in exon 19 (except the deletion mutation in exon 19, n=26) and exon 21 (except the L858R mutation n=3), and others (n=4). No further subgroup analysis was performed because of the limited sample sizes.
5. Relationship between PD-L1 expression and RFS and OS
During a median follow-up of 83 months, 128 disease recurrences and 51 deaths occurred. RFS was significantly shorter (p < 0.001, log-rank test) in the PD-L1 TPS ≥ 50% (median, 17.36 months; 95% confidence interval [CI], 13.9 to 42.7) and PD-L1 TPS 1%-49% groups (median, 31.6 months; 95% CI, 28.1 to 39.0) compared with the PD-L1–negative group (median, 35.5 months; 95% CI, 35.0 to 55.9) (Fig. 2). Using adjusted Cox model 1 and compared with the PD-L1–negative group, the adjusted hazard ratio (HR) for RFS was 1.60 (95% CI, 0.88 to 2.93) for those with PD-L1 TPS ≥ 50%, 1.49 (95% CI, 1.02 to 2.18) for those with PD-L1 TPS 1%-49%, and 1.51 (95% CI, 1.05 to 2.18) for the combined PD-L1–positive groups (TPS ≥ 1%) (Table 2). The results were similar after adjusting for adjuvant chemotherapy or radiotherapy (Cox model 2), with an adjusted HR of 1.52 (95% CI, 0.81 to 2.84) for those with PD-L1 TPS ≥ 50%, 1.51 (95% CI, 1.02 to 2.21) for those with PD-L1 TPS 1%-49%, and 1.51 (95% CI, 1.04 to 2.19) for the combined PD-L1–positive groups (Table 2).
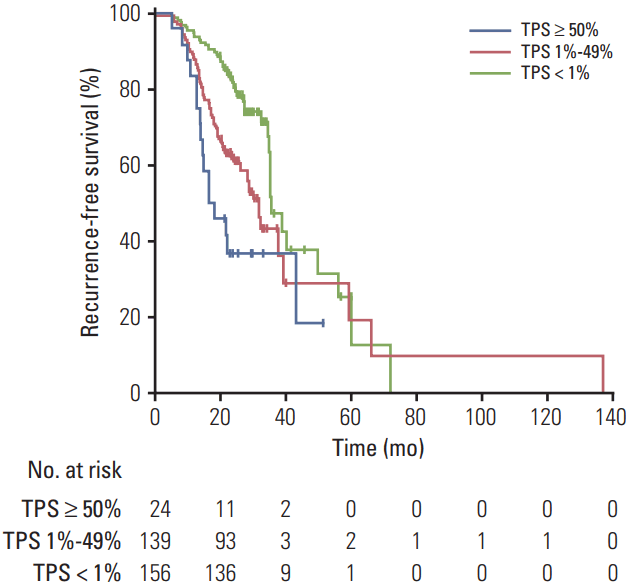
Recurrence-free survival among patients with nonsmall cell lung cancer and epidermal growth factor receptor mutation, by programmed death ligand 1 status. TPS, tumor proportion score.
PD-L1 TPS ≥ 50% was associated with shorter OS (p=0.077, log-rank test) in the crude analysis (Table 2, Fig. 3), with a crude HR of 2.70 (95% CI, 1.07 to 6.66) for PD-L1 TPS ≥ 50% (median OS, 64.5 months; 95% CI, 34 to not reached) and 1.04 (95% CI, 0.57 to 1.89) for PD-L1 TPS 1%-49% (median OS, 87.8 months; 95% CI, 66.6 to 123.9), when compared with the PD-L1–negative group (median OS, 76.6 months; 95% CI, 68.6 to 89.5). However, the association was not statistically significant in either adjusted Cox model 1 or 2 (Table 2).
Discussion
In the current study, which included predominantly patients with early-stage EGFR-mutant NSCLC, 51% of patients had PD-L1–positive tumors, suggesting that PD-L1 is commonly expressed even among patients with EGFR-mutant early-stage NSCLC. Consistent with our previous study [23], a higher prevalence of PD-L1 positivity was observed among men, smokers, and patients with advanced disease. Patients with EGFR exon 19 deletion or exon 21 L858R mutation were less likely to have PD-L1–positive tumors than patients with other EGFR mutations, suggesting that PD-L1 expression may be associated with different types of EGFR mutation status. The exact reason for the association between PD-L1 expression and different types of EGFR mutations is not clear, although both PD-L1 expression and types of EGFR mutations are associated with clinical characteristics such as sex, smoking status, and disease stage [24-26].
At this point, data are limited regarding the prognostic effect of PD-L1 among patients with EGFR mutations. The correlation between tumor PD-L1 status and EGFR mutation status has been studied previously, with contradictory results [24-26]. One study showed a higher EGFR mutation rate in patients with lower PD-L1 expression and that the presence of EGFR mutation increased OS, whereas high PD-L1 expression decreased OS [24]. However, this study did not explore the relationship of uncommon EGFR mutations. Rather, it focused on the effect of the presence or absence of EGFR mutations and PD-L1 expression [24]. Another study showed that there was no significant relationship between PD-L1 expression, common baseline characteristics, and EGFR mutation, but there was an association of PD-L1 expression with response to EGFR TKI [26]. Furthermore, in the group of patients with EGFR mutations, PFS and OS of patients with PD-L1–positive tumors tended to be longer than in patients with PD-L1–negative tumors; however, statistical significance was not achieved [26]. This may have been due to the smaller sample size (n=170) in the Tang et al.’s study [26] compared with the current study.
It has been postulated that EGFR activation promotes expression of PD-L1 by the nuclear factor κB pathway [27]. This pathway may underlie acquired resistance to EGFR TKIs [27]. Thus, combined targeted therapy using immune checkpoint inhibitors and EGFR TKIs may provide a novel therapeutic option for patients with EGFR-mutant NSCLC [27]. Preliminary results from the CheckMate 012 study in patients with advanced NSCLC reported that nivolumab 3 mg/kg intravenously every 2 weeks and erlotinib 150 mg orally daily enabled 15% of TKI-refractory patients to achieve a partial response (3 of 20) [28]. Ongoing clinical trials that are investigating the combination of immune checkpoint inhibitor and EGFR TKI include a phase I study investigating the recommended phase II dose of the immune checkpoint inhibitors nivolumab or ipilimumab and erlotinib or crizotinib in patients with advanced NSCLC and EGFR mutations or ALK translocations (no longer enrolling; ClinicalTrials.gov identifier, NCT01998126) and a phase I study of pembrolizumab and afatinib in patients with advanced NSCLC (currently enrolling; ClinicalTrials.gov identifier, NCT02364609).
The prognostic implications of PD-L1 expression and OS have been inconsistent, with studies or meta-analyses providing negative and positive associations [9,29,30]. In the current study, we observed a trend of negative association between PD-L1 expression and OS, particularly in the subgroup of patients with TPS ≥ 50%; however, the result was not statistically significant after adjusting for other baseline characteristics. Part of the reason is because of the good prognosis of this patient population (e.g., patients with EGFR-mutant, early-stage adenocarcinoma) and relatively shorter follow-up, and only 51 deaths were observed during the median follow-up of 83 months. Our study suggests that positive PD-L1 expression may be associated with short RFS.
The strengths of this study include a homogeneous population of patients with NSCLC and EGFR mutations, relatively large sample size, and complete clinical and outcome information. In addition, we used the FDA-approved companion diagnostic immunohistochemical assay (PD-L1 IHC 22C3 pharmDx), which is the same as that used in pembrolizumab clinical trials. Limitations to this study include the retrospective nature of the analysis, small number of patients with stage IV disease, and lack of comparison with patients with wild-type EGFR. In addition, 97% of the patients had adenocarcinoma, and we were not able to examine the association between PD-L1 expression and survival among other histologic subtypes of patients.
In conclusion, our results suggest that PD-L1 is commonly expressed among patients with early-stage NSCLC with EGFR mutations, and higher PD-L1 expression may be associated with short RFS for patients with EGFR-mutant NSCLC. Notably, the type of EGFR mutation may affect the expression of PD-L1, although this does not appear to have an effect on survival. The relationship of these results with response to currently available therapies targeted to EGFR or PD-1/PD-L1 remains to be investigated.
Notes
This study was supported by Merck & Co., Inc. (Kenilworth, NJ, USA). Editorial assistance was provided by Jennifer M. Kulak, PhD, of the ApotheCom oncology team (Yardley, PA, USA) and was funded by Merck & Co., Inc., Kenilworth, NJ, USA. Drs. Cho, Y.-L. Choi, Sun, H. Choi, T.-E. Kim, and J. Kim have no conflicts to disclose. Drs Zhou, Dolled-Filhart, Emancipator, and Rutkowski are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and own stock in the company. Dr. Emancipator also has stock in Bayer AG and Johnson and Johnson and his spouse is employed by and has stock ownership in Celgene.
Acknowledgements
The authors thank Eric Rubin, Gregory M. Lubiniecki, Cong Chen, Jared Lunceford, Jun Hun, Melissa Whipple, and Qing Shao (all of Merck and Co., Inc., Kenilworth, NJ, USA).