Analysis of the Clinicopathological Characteristics of Gastric Cancer in Extremely Old Patients
Article information
Abstract
Purpose
Gastric cancer is the third-leading cause of cancer-related death in Korea. As the Korean population is ageing, the number of extremely old patients with this disease is increasing. This study examined the clinicopathological characteristics of gastric cancer in extremely old (over 85 years) patients who received treatment or conservative observations and compared the treatment outcomes according to the treatment modality.
Materials and Methods
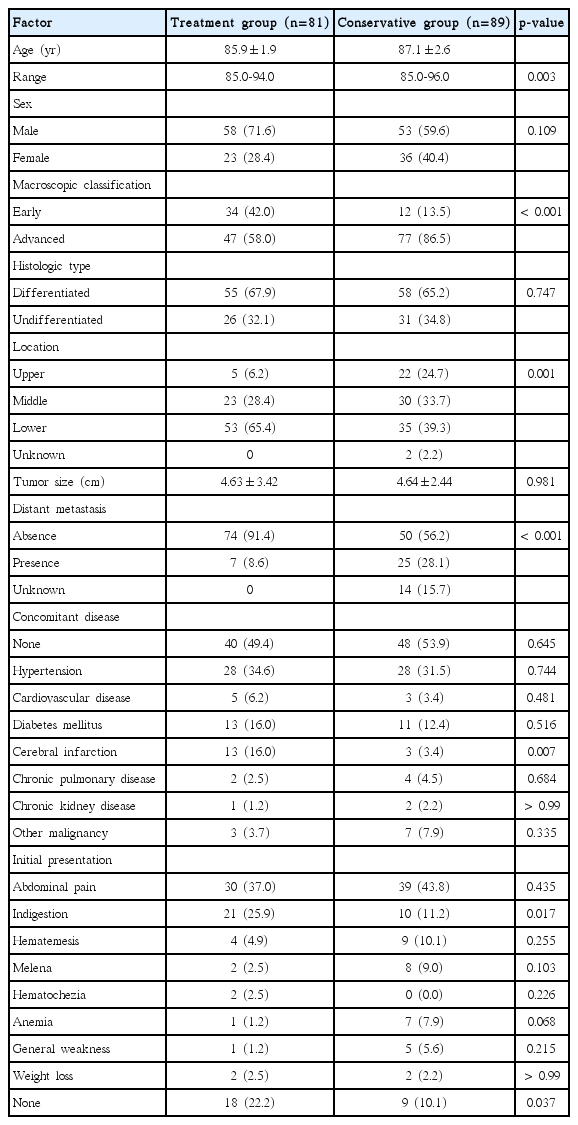
A total of 170 patients over 85 years of age were diagnosed with gastric cancer. Of these, 81 underwent treatment for gastric cancer and 89 received conservative observations. The clinicopathological characteristics of the treatment and conservative groupswere compared.
Results
The mean age of the patients was 86.5 years. The conservative group included significantly more patients with older ages, macroscopically advanced cancer and upper-middle located cancer. The overall survival rate of the treatment group was significantly higher than that of the conservative group. The disease-specific mortality rate was significantly lower in the treatment group than in the conservative group. Multivariate analysis revealed the clinical course, alarm sign, and macroscopic classification to be independent prognosis factors.
Conclusion
By itself, the chronological age should not be used as a strategy to determine whether treatmentwill be administered for gastric cancer. Patients who have early gastric cancer or lower-risk preexisting comorbidities should not be discouraged from treatment, even if they are older than 85 years.
Introduction
Following thyroid cancer, gastric cancer (GC) is the second most common cancer in Korea [1]. Recently, as the Korean society is aging, there has been an increasing number of elderly patients diagnosed with GC. Despite this, there is little data available on the clinicopathological characteristics of GC in elderly patients, particularly in those who are very elderly. In Korea, the life expectancy is more than 80 years and the most common cause of death is cancer [2]. According to the statistics released by Statistics Korea, approximately 12% of cancer-related deaths have been caused by GC in recent years. Although the 5-year survival rate of GC has been increasing over the past two decades due to early detection by screening endoscopy [3], little is known about GC screening in the very elderly.
Endoscopic treatment is the accepted standard treatment for selected cases of GC. The safety of endoscopic treatment is comparable to surgery in elderly patients [4-8]. Endoscopic resections (ERs) are also performed on elderly patients with comorbid heart and lung diseases [9]. Therefore, the strategy of GC treatment varies in elderly patients with age-associated comorbidities. Age is a clinically important factor for determining the treatment modality [10], and clinicians are often cautious when managing elderly patients with GC because of their age-related performance status [11-13].
This study investigated the clinicopathological characteristics of GC in extremely old (over 85 years) patients who received treatment or conservative observations. In addition, the treatment outcomes were compared according to the treatment modality.
Materials and Methods
1. Patients
From June 1998 to July 2014, a total of 358 patients over 85 years old were diagnosed with GC at Severance Hospital (Seoul, Korea). For the present analysis, the following patients were excluded (1) those who had no subsequent follow-up visits after diagnosis, (2) those who had a significant comorbidity that could affect the mortality, and (3) those who had been treated at another hospital during the follow-up period. Of the 358 patients, 188 were excluded and the remaining 170 were enrolled. The patients were evaluated in terms of their general information, such as age, sex, and treatment method. The variables investigated also included the macroscopic classification, histological classification, comorbidity, postoperative mortality, and clinical outcome. As categorized by the treatment modality for GC, 81 patients underwent treatment (treatment group), such as an endoscopic resection, surgery, chemotherapy, or radiotherapy, and 89 patients received conservative observation (conservative group). The staging of the patients in the conservative group could not be evaluated accurately. Therefore, the patients were divided roughly into two groups: those with macroscopically early GCs (cancer confined to the mucosa or submucosa) and those with macroscopically advanced GC (cancer with invasion extending through the muscularis propria). The patients’ medical records were reviewed retrospectively. The Institutional Review Board (IRB) of Severance Hospital approved this study (IRB approval number: 4-2014-0464).
2. Statistical analysis
The chi-square test and Fisher exact test were used to compare the clinicopathological characteristics between the treatment-modality groups. Follow-up data on all patients were obtained from the Korea National Health Insurance Service database. The median follow-up period was 17.8 months (range, 0.1 to 172.4 months). The survival curves were estimated using the Kaplan-Meier method. The accepted significant level was p < 0.05. All statistical analyses were performed using SPSS ver. 20.0 for Windows (IBM Co., Armonk, NY).
Results
1. Clinicopathological characteristics
The sex or histologic type was similar in the two groups. On the other hand, older age, macroscopically advanced cancer, and upper-middle located cancer were significantly more common in the conservative group (Table 1). In the treatment group, 21 patients underwent ER, 48 patients underwent gastrectomy, and 12 patients underwent chemotherapy or radiotherapy. Of the 21 patients who received ER, 20 had lesions included in the absolute indication and one patient had lesions included in the expanded indication. Of the 48 patients who received surgery, 46 underwent a curative gastric resection (subtotal gastrectomy, n=43; total gastrectomy, n=3) and two received palliative gastrectomy. The curative resection rate was 18/21 (85.7%) for ER and 46/48 (95.8%) for gastrectomy. In the conservative observation group, 38/89 patients (42.7%) refused the recommended treatment.
In the overall study cohort, the most common concomitant disease was hypertension (32.9%). Eighty-two patients (48.2%) had one or more comorbid diseases and 37 patients (21.8%) had more than two comorbid diseases.
One hundred forty-three patients (84.1%) visited the hospital with symptoms and 27 patients (15.9%) were diagnosed with GC during a health check-up. The most common initial presentation was abdominal pain, which was present in 69 patients (40.6%). Thirty-seven patients (21.8%) had alarm signs (weight loss, signs and symptoms of upper gastrointestinal bleeding, and anemia), and the presence of an alarm sign was significantly more common in the conservative observation group than in the treatment group. On the other hand, indigestion was significantly more common in the treatment group.
2. Clinical outcomes
A postoperative complication (bleeding) occurred in one patient who underwent a gastrectomy; this patient died three days after surgery. No other procedure-related complications were observed in the patients who received ER. Recurrence developed in two of the patients who received ER. One patient had a local recurrence and the other patient developed liver metastasis. Eight of the patients who underwent surgery experienced a recurrence.
3. Survival
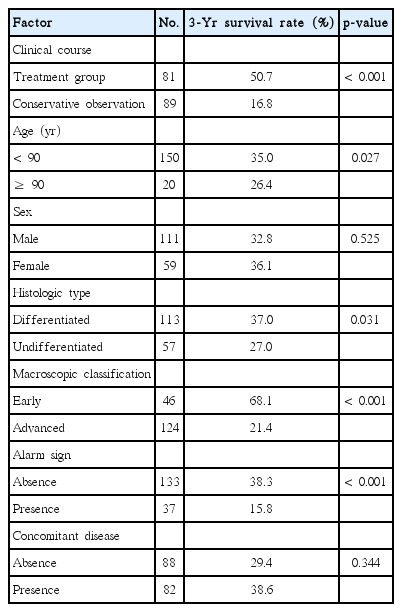
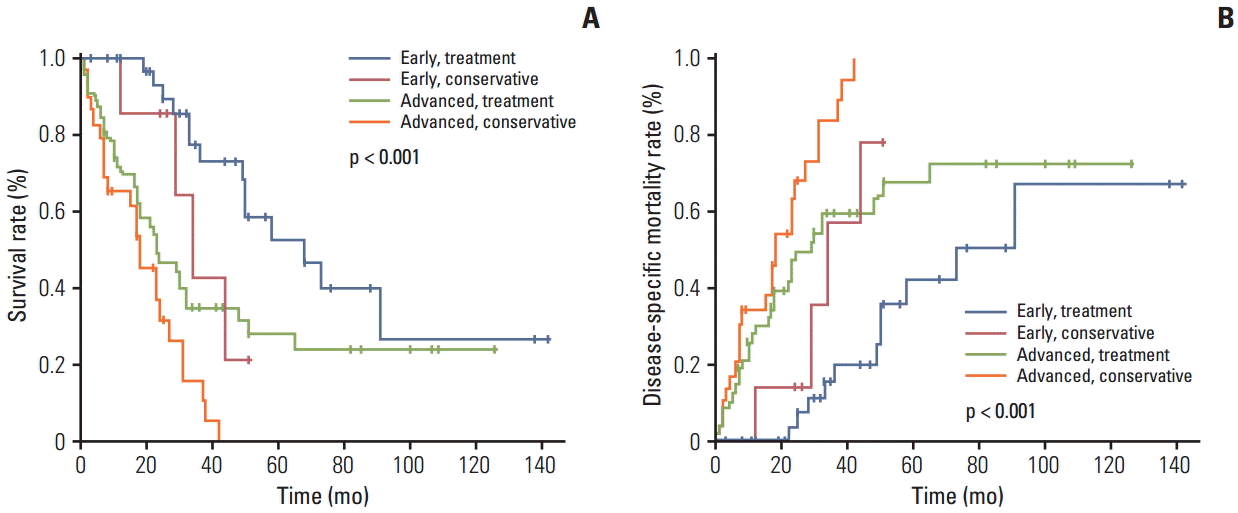
The overall survival rate in the treatment group was significantly higher than that in the conservative group (Fig. 1A). Furthermore, the disease-specific mortality was significantly lower in the treatment group than in the conservative group (Fig. 1B). The 81 patients in the treatment group and the 89 patients in the conservative group were divided into early GC and advanced GC subgroups, as shown in Fig. 2. For the patients with advanced GC, the overall survival rate was significantly higher in the treatment group than in the conservative group. For patients with early GC, the overall survival rate was also significantly higher in the treatment group than in the conservative group (p < 0.001) (Fig. 2A). Similarly, the disease-specific mortality in the treatment group was significantly lower than that in the conservative group, regardless whether early or advanced GC had been diagnosed (p < 0.001) (Fig. 2B). Subgroup analysis was performed on the overall survival and disease specific mortality between the conservative observation group who refused the recommended treatment and the treatment group. The overall survival rate of the treatment group was significantly higher than the conservative observation group who refused the recommended treatment and the disease specific mortality rate was significantly lower (Fig. 3). The patients in the treatment group and the conservative observation group who refused the recommended treatment were divided into early GC and advanced GC subgroups. The overall survival rate was significantly higher and the disease-specific mortality rate was significantly lower in the treatment group than in the conservative observation group who refused the recommended treatment, regardless whether early or advanced GC had been diagnosed (Fig. 4). In addition, the survival according was evaluated to treatment modality. The overall survival rate of the ER and surgery group was similar and the disease-specific mortality of the ER and surgery group was also similar (data not shown).
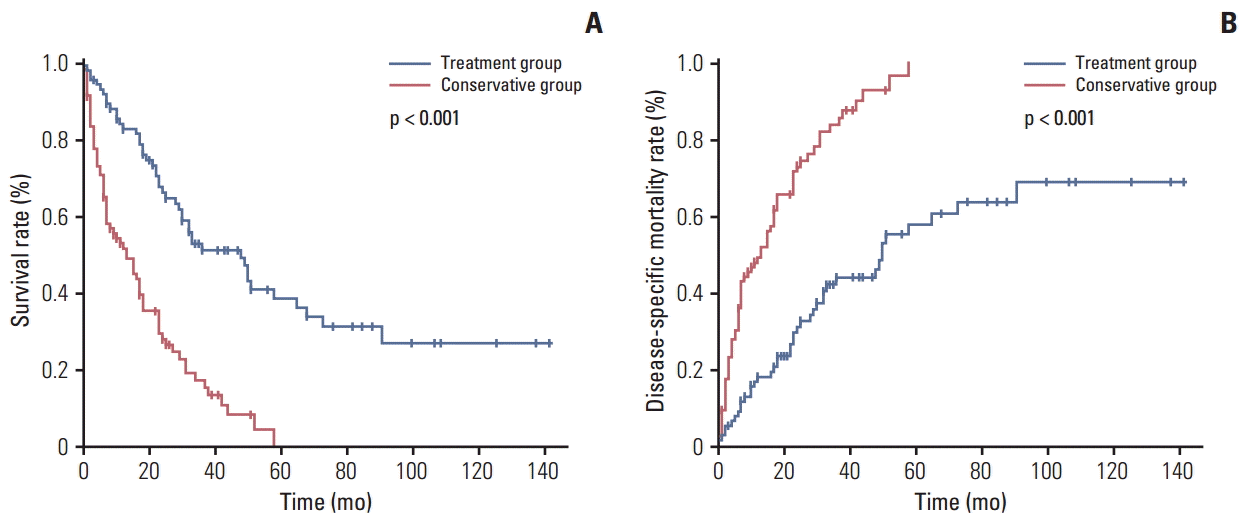
(A) Overall survival rate of the treatment (n=81) and conservative (n=89) groups. (B) Disease-specific mortality of the treatment (n=81) and conservative (n=89) groups.
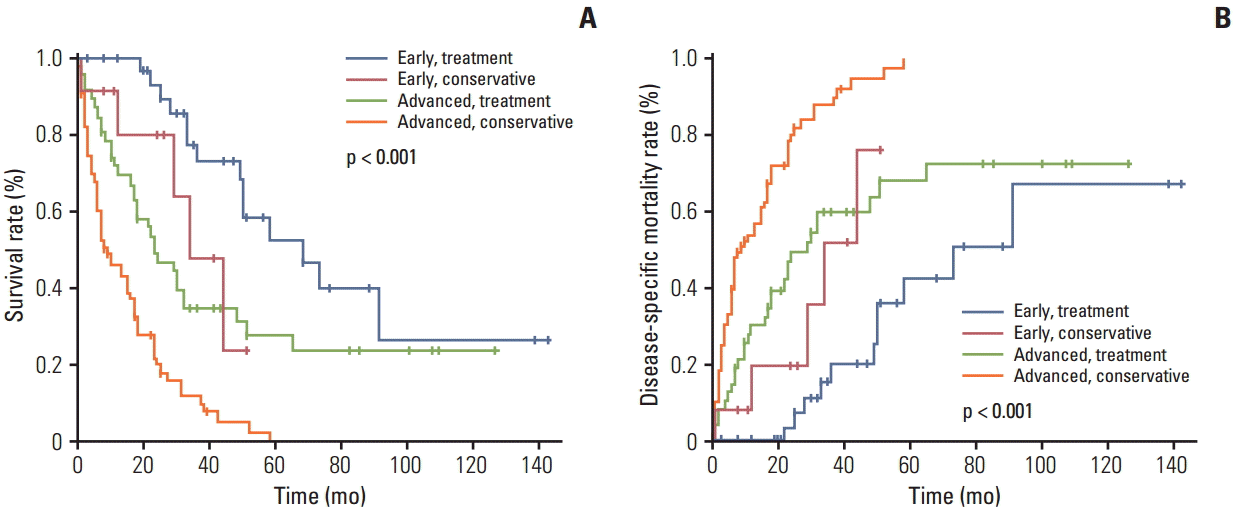
The patients in the treatment group and the conservative group were divided into early gastric cancer (GC) and advanced GC subgroups (early and treatment group, n=34; early and conservative group, n=12; advanced and treatment group, n=47; and advanced and conservative group, n=77). (A) Overall survival rate with GC patients showing macroscopic classification and clinical course. (B) Disease-specific mortality rate with GC patients showing macroscopic classification and clinical course.

(A) Overall survival rate of the treatment (n=81) and conservative (n=38) groups refused recommended treatment. (B) Disease-specific mortality of the treatment (n=81) and conservative (n=89) groups refused recommended treatment.
The patients in the treatment group and the conservative group refused recommended treatment were divided into early gastric cancer (GC) and advanced GC subgroups (early and treatment group, n=34; early and conservative group, n=9; advanced and treatment group, n=47; and advanced and conservative group, n=29). (A) Overall survival rate with GC patients showing macroscopic classification and clinical course. (B) Disease-specific mortality rate with GC patients showing macroscopic classification and clinical course.
4. Effects of clinicopathological characteristics on prognosis
Seven factors (clinical course, age, sex, histologic type, macroscopic classification, alarm sign, and concomitant disease) were evaluated by univariate analyses to investigate the associations of the clinicopathological characteristics with the prognosis. Significant differences were observed for the clinical course (p < 0.001), age (p=0.027), histologic type (p=0.031), alarm sign (p < 0.001), and macroscopic classification (p < 0.001) (Table 2). The same seven factors were then analyzed by multivariate analysis, which revealed the clinical course (p < 0.001), alarm sign (p=0.009), and macroscopic classification (p < 0.001) to be independent prognosis factors (Table 3).
Discussion
As the society ages, clinicians are increasingly being confronted with the challenges of treating elderly patients. In Korea, the number of patients diagnosed with GC at extremely old ages has been increasing [1]. Several reports have shown that older patients tend to receive less treatment for GCs than younger patients [14-18]. When clinicians make the decision to treat cancer, they consider the cancer stage, the patient’s performance status, deterioration of the patient’s mental status, and concomitant disease together; they do not rely on the patient’s age alone. Generally, the prognosis of elderly patients has been thought to be worse than that of younger patients because of concomitant disease. On the other hand, deaths due to other concomitant diseases amounted to 34%-37% of the total deaths in > 80-year-old patients with GC [11,19]. Matsushita et al. [20] investigated patients with GC who were older than 80 years and reported that the survival rate after surgical treatment was significantly higher than that for conservative observation, regardless of the patient’s performance status and irrespective of whether his or her mental status had deteriorated. The macroscopic classification of the case (e.g., early or advanced cancer) was an important prognostic factor. Therefore, they recommended surgical treatment for patients older than 80 years [20]. Treatments are recommended for GC, even for older patients with age-associated comorbidities that are under control [21]. In a study of the natural history of early GC, Tsukuma et al. [22] reported that the cumulative 5-year risk for progression to an advanced stage was 63.0%. Even patients with early GC usually die within 3 years in the absence of treatment [20]. On the other hand, Katai et al. [23] reported that when a gastrectomy had been performed safely by specialists, the survival rate of elderly patients with early GC was not significantly different from that of the general population. Therefore, the treatment of early GC should not be discouraged in very elderly patients. In this study, patients in the treatment group, those with early GC, and those without an alarm sign showed higher overall survival rates than the others.
Currently, ER is widely accepted as a standard treatment for early GC [24]. Compared to gastrectomy, ER is a less invasive treatment for GC and has many advantages. These advantages include preservation of the stomach, which increases the quality of life and reduces the length of hospitalization compared to gastrectomy. Furthermore, ER-related complications result from the difficulties encountered when performing the resection endoscopically, which are associated with the tumor size and location, rather than age [6,8]. Several reports have shown that ER is a safe and feasible treatment for GC in elderly patients [5,6,8]. Etoh et al. [25] reported that the results of an endoscopic mucosal resection in patients older than 80 years were outstanding, and were the same as those obtained via gastrectomy. Therefore, elderly patients with earlier-stage cancer should receive less-invasive treatments. As the techniques of preoperative and perioperative management have improved, the outcomes of gastric surgery in the elderly have also shown favorable results [23,26]. Therefore, treatment should not be discouraged for elderly patients with GC. Similarly, the overall survival rate of the patients in the present study’s treatment group was significantly higher than that in the conservatively observed group.
In this study, advanced GC accounted for 72.9% of cases. The percentage of patients with advanced cancer was significantly higher in the conservative observation group than in the treatment group. Advanced-stage cancers were a cause of the poor prognosis. Therefore, it is important to recognize cancers at the earlier stage to provide a better prognosis in elderly patients. In a previous study, the prevalence of preexisting concomitant disease was significantly higher in elderly patients [8]. In the present study, 88 patients (51.8%) did not have any concomitant disease. Furthermore, 143 patients (84.1%) presented with symptoms and 37 patients (21.8%) showed an alarm sign. In addition, patients with an alarm sign had advanced-stage cancers. Therefore, elderly patients who are being followed up for concomitant disease also need to be checked for gastrointestinal disease. When patients have symptoms, the clinicians need to consider further evaluations.
This study had some limitations. This study was retrospective and could not investigate exactly how the decision was made as to whether to treat the patients or not. In addition, the TNM classification of the patients could not be evaluated because the staging of the patients in the conservative group could not be investigated accurately. In this study, however, the overall survival of the patients with advanced GC was significantly lower than in the early GC. Furthermore, the macroscopic classification was an independent prognosis factor in multivariate analysis.
Conclusion
Chronological age alone should not be used as a strategy of determining if treatment will be administered for GC. Patients with early GC or lower-risk preexisting comorbidities should not be discouraged from treatment, even if they are older than 85 years.
Notes
Conflict of interest relevant to this article was not reported.