Age-Period-Cohort Analysis of Female Breast Cancer Mortality in Korea
Article information
Abstract
Purpose
Despite the low mortality rate of breast cancer among women in Korea, the breast cancer mortality rate has increased. The aim of this study was to examine trends in breast cancer mortality from 1983 to 2012 in Korea, assessing the importance of age, period, and birth cohort as risk factors.
Materials and Methods
Data on the annual number of deaths due to female breast cancer and on female population statistics from 1983 to 2012 were obtained from Statistics Korea. A log-linear Poisson age-period-cohort model was used to estimate age, period, and cohort effects.
Results
The increasing breast cancer mortality can be explained predominantly by a birth cohort effect: the risk of breast cancer death showed a steady increase until the 1968 birth cohort, and decreased thereafter. There was a sharp increase in the magnitude of the age effect up to 60 years old, then a moderate increase in the effect during the sixties, followed by another sharp increase from 70 years old. The period effect on breast cancer mortality seems negligible based on its adjusted relative risk, even though it was statistically significant after adjusting for age and cohort effects.
Conclusion
In this study, the mortality pattern of breast cancer in Korea can be explained predominantly by a birth cohort effect. Hence, the overall mortality rate of breast cancer may increase for a while, and show a gradual decrease in the future, which will start from the younger age group.
Introduction
In Korea, the breast cancer mortality rate among women is relatively low compared to the high incidence rate of this cancer. The crude incidence and mortality rates were 63.7 and 8.0 per 100,000 in 2011, respectively [1]. Female breast cancer is the second most common malignancy, and its incidence has ranked second to thyroid cancer in Korea since 2004, while female breast cancer mortality ranked sixth in 2011 [1].
Nevertheless, the long-term mortality rate of breast cancer among women in Korea has shown a steady increase, from 2.1 per 100,000 in 1983 to 7.9 per 100,000 in 2012 [2]. This increasing trend might be attributed to higher prevalence of westernized dietary habits, postmenopausal obesity, and reproductive risk factors such as lower fertility rate among Korean women. Several of these changes are likely to continue in parallel with economic growth, thus further increases in breast cancer incidence seem inevitable [3,4].
Investigations of secular trends in breast cancer incidence and mortality rates contribute clues regarding the etiology of the illness. Several studies have examined birth cohort effects on breast cancer incidence and mortality rates [5,6]. However, this is the first study to evaluate a birth cohort effect on breast cancer mortality in Korea.
In the current study, age-period-cohort analysis was performed using female breast cancer mortality data in Korea from 1983 to 2012, with the aim of determining the potential role of the birth cohort effect on the reported increase in breast cancer deaths among Korean women.
Materials and Methods
Data on the annual number of deaths due to female breast cancer and on female population statistics from 1983 to 2012 were obtained from Statistics Korea (Table 1) [2]. Because breast cancer deaths in individuals under 20 years old are rare, the analysis was restricted to death at aged between 20 and 79 years, and was grouped according to 5-year intervals. Age-adjusted mortality rates were calculated using the female population in 1997 as a standard. Breast cancer cases were defined as code C50 (malignant neoplasm of breast) in accordance with the International Classification of Diseases 10th revision (ICD-10). To compare the trends in the mortality rate with the incidence rate, the age-specific female breast cancer incidence in Korea from 1999 to 2011 was obtained from Statistics Korea [2].
Log-linear models were employed for evaluation of the effects of age, period, and birth cohort on female breast cancer mortality. Because age, period and cohort are continuous variables, their impact on the death rate was analyzed in a continuous-time model. A nonlinear regression model was estimated with the mortality rate as a dependent variable observed for each age, year of death, and birth cohort. The formulation of the multiplicative age-period-cohort model for mortality rate r(a,p) at age a in period p for persons in birth cohort c=p–a is:
, where fco(a) are the age-specific rates in the reference cohort c0=1948 (mid-point of the cohorts); δ is the slope of the log-linear trend in cohort (drift); h(c) is the cohort function, interpretable as log relative risk to cohort c0, and g(p) is the period function.
There is a fundamental problem of non-identifiability in age-period-cohort models. To obtain identifiability, h(c) and g(p) are de-trended, with a zero slope, and the average period function is zero. The inclusion of the drift with the cohort effect makes the age effect interpretable as a cohort-specific rate of mortality. The drift was extracted using the weight method. Using this model the effects of age, period, and birth cohort are nonlinear through the functions fco(a), h(c), and g(p). Each of the three functions was parameterized using a natural spline with five knots.
Based on the general form, we established five models in sequence: a one-factor age model, a two-factor age-drift model, an age-period model, an age-cohort model, and finally an age-period-cohort model. The drift term in the age-drift model represents a temporal change in mortality rate not identifiable as a period or cohort effect. By comparing deviances from the models, the statistical significance of each factor was tested after adjusting for the others. Because age is a significant predictor of cancer mortality, a goodness of fit considering age would be valuable. Therefore, age-adjusted R2A was used to evaluate goodness of fit [7], as it measures how much variability can be explained by factors other than age. For example, the variability contribution by period is given by:
, where D and df are the model deviance and degree of freedom, respectively.
The same data between 1983 and 2012 were applied for prediction of breast cancer deaths using the method based on that of Kristensen [8]. It assumed that each estimated cohort effect was constant over the past and future years. This assumption becomes difficult to maintain when the predicted year is far from actual data. Hence, the ex-post forecast was limited to 1970 and the ex-ante forecast was limited to 2020. The forecasting model of death rate became
, where A, P, and Ci are age at death, year of death, and dummy variable for cohort i.
The analyses were performed using the R programming language using the apc.fit function from the Epi package (http://www.r-project.org) and SAS ver. 9.3 (SAS Institute, Cary, NC) [9].
Results
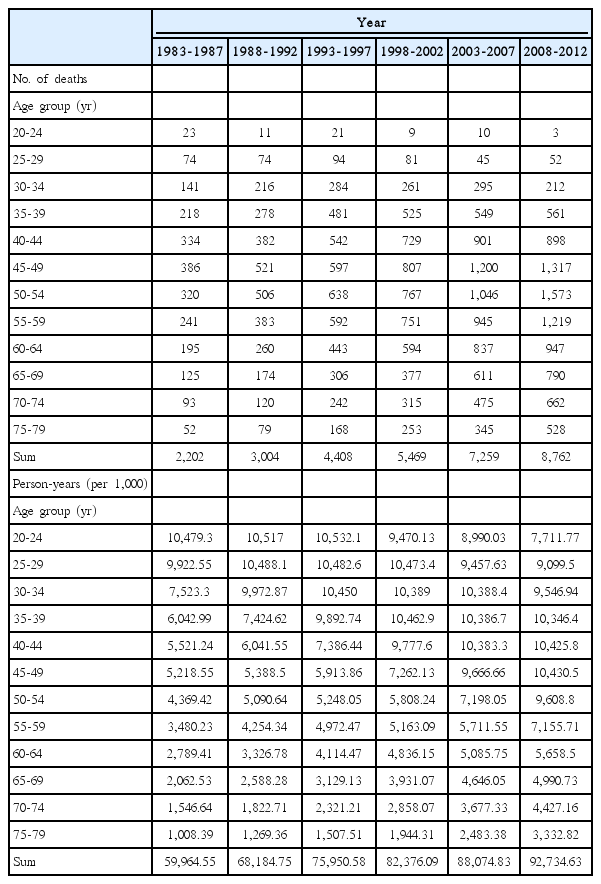
Breast cancer deaths increased according to calendar years, and total number of breast cancer deaths among Korean women was 31,104 from 1983 to 2012 (Table 1). Female breast cancer mortality rates in different time periods are depicted in Fig. 1. Age-specific breast cancer mortality rates were higher in the more recent periods compared to those in the older periods (Fig. 1A). Within each period, overall, mortality rates increased until the ages of 40-50 years and then decreased. However, the specific patterns differed by periods: the age group of the peak mortality shifted to an older age group in the recent periods, and at the same time, the mortality in the age group of over 70 years old decreased in the older periods (1983-1987 and 1988-1992) but increased in recent periods (from 1993-1997 to 2008-2012). Such different patterns in cross-sectional age curves by time period may imply a birth cohort effect. The age-specific mortality trends by birth cohorts increased in recent birth cohorts (Fig. 1B). In particular, the increase was more evident in the older age groups. Within each birth cohort, the mortality rates of breast cancer increased overall with age. However, mortality rates in the ‘60-64’ age group (age of 62 in Fig. 1B) and in the ‘65-69’ age group (age of 67 in Fig. 1B) were similar.
Table 2 shows the goodness of fit (scaled deviance) for the age-period-cohort models. The age-drift model gave a lower value of adj–R2A than that for the age-cohort or age-periodcohort models, thus it was excluded from the possible models of mortality in breast cancer. After adjusting for age, the period effect and cohort effect were statistically significant (p < 0.0001). Based on adj–R2A , the age-cohort model seems to explain the death rates better than the age-period model and similarly to the full model, which implies that breast cancer mortality can be mainly explained by a birth cohort effect rather than a period effect. The period effect adjusted for age and birth cohort effect and the cohort effect adjusted for age and period were also statistically significant (p < 0.0001). All factors in the age-period-cohort model were statistically significant after adjusting for each other. Therefore, the full model was used to represent breast cancer mortality.

Summary statistics of various age-period-cohort models for female breast cancer mortality in Korea between 1983 and 2012
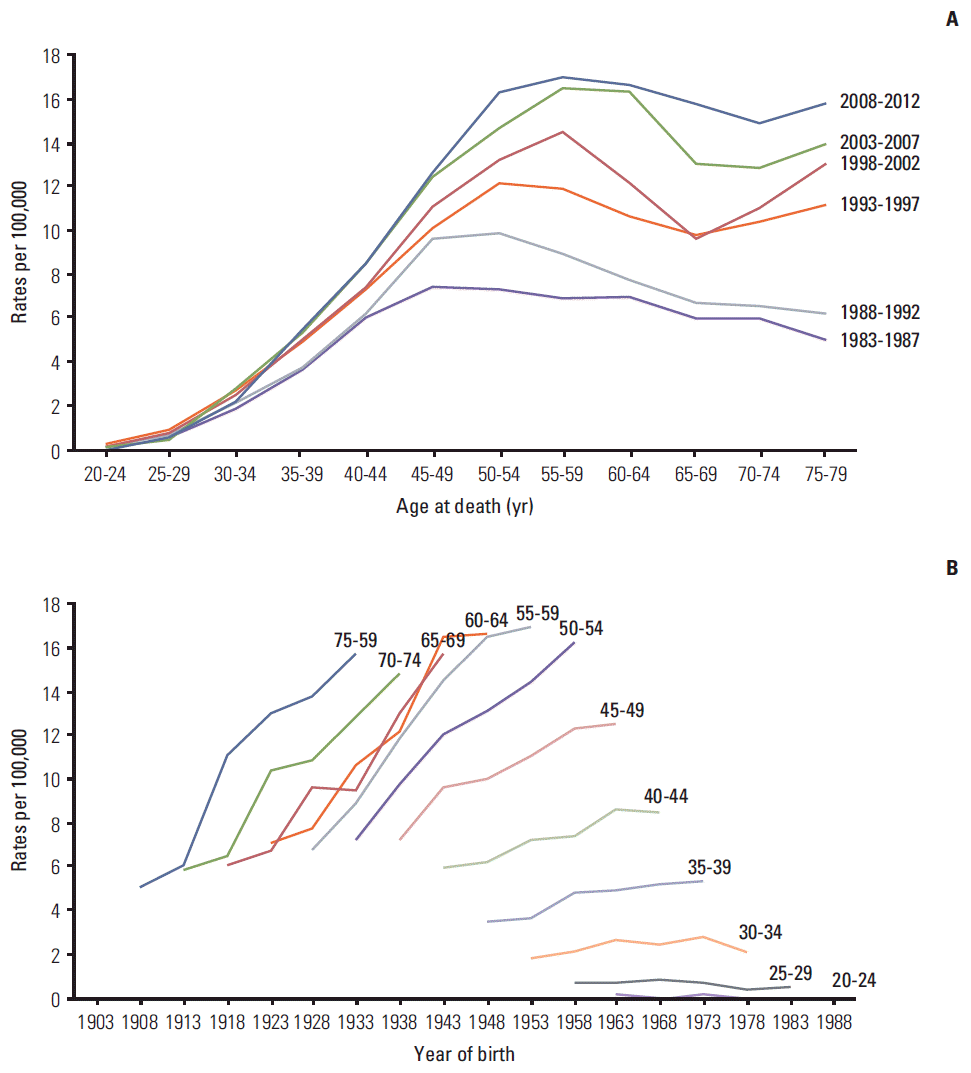
The mortality trends according to age, birth cohort, and period were adjusted for the other two parameters shown in Fig. 2. In the graph of mortality trends by age (Fig. 2A), the rate increased sharply until 60 years old, showed a moderate increase in the sixties, and a steep increase again from 70 years old. This pattern is consistent with the pattern examined in Fig. 1B. The cohort effect increased in cohorts born before 1968 and decreased in cohorts born after 1969 ([a] in Fig. 2B).

Age-period-cohort effect on female breast cancer mortality in Korea. The left graph shows the mortality rate per 100,000 by age for the reference cohort (birth year 1948) (A); the two right graphs show mortality risk (B) by birth cohort (a) and period (b) (dotted line represents 95% confidence interval).
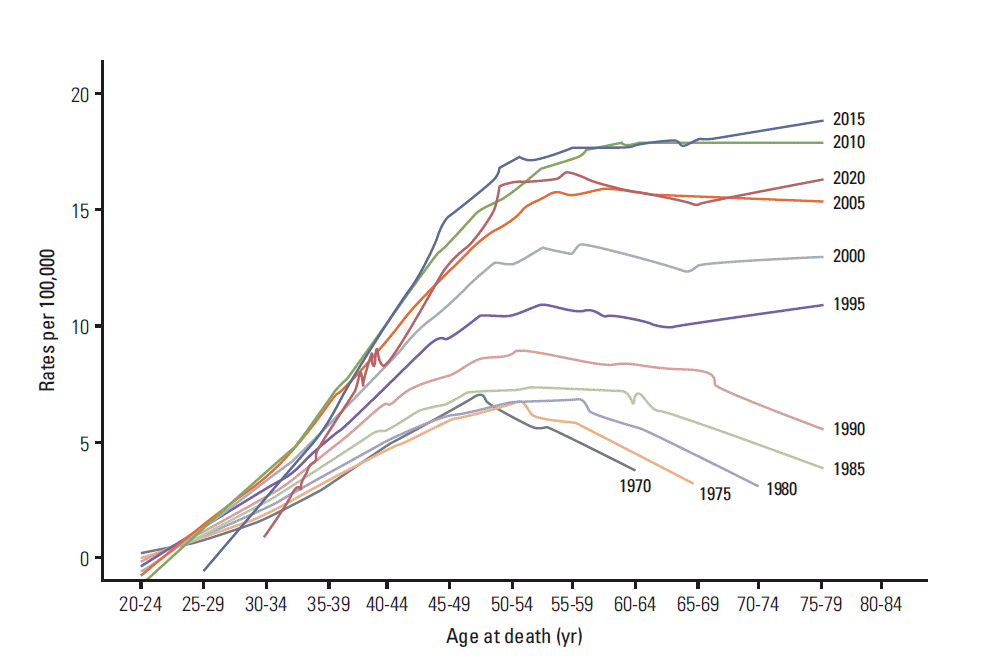
The predicted mortality trends by age are shown in Fig. 3. The mortality rates increase by period until the year 2015 and then decrease by the year 2020. The mortality trends by age showed an increasing and decreasing pattern before the year 1995, but an increasing pattern from the year 1995. However, Clemmensens’ hooks were observed in all curves.
The age-specific female breast cancer incidence rate in Korea from 1999 to 2010 is shown in Fig. 4. The highest incidence rate was observed between the ages of 45-49 in all calendar years. The breast cancer incidence in the thirties age group showed a moderate increase during 1999-2011, which corresponds to the cohorts after 1969. In addition, the annual percentage change in breast cancer incidence from 1993-2002 was estimated as 6.0% in the 20-34 age group [10], which approximately corresponds to the birth cohort after 1969. These data imply that the decreasing mortality risk after the birth cohort of 1969 was not caused by a decrease in breast cancer incidence.
Discussion
In our age-period-cohort analysis of breast cancer mortality among women in Korea from 1983 to 2012, the increasing rate of breast cancer mortality can be explained predominantly by a birth cohort effect: the risk of breast cancer death showed a steady increase until the 1968 birth cohort, and decreased among women born after 1969. Between 1983 and 2012, breast cancer death in Korea decreased by 4% (95% confidence interval [CI], 1% to 7%; p=0.0021) among women younger than 30 years old, while it increased by 2.5% (95% CI, 2.3% to 2.7%; p < 0.0001) and by 3.5% (95% CI, 3.3% to 3.8%; p < 0.0001) in the age groups of 30-54 and 55-79, respectively. Since the risk of breast cancer mortality is lower in the recently born generation and the breast cancer mortality is predominantly explained by the birth cohort effect, it is speculated that breast cancer death will probably decrease in Korea (Fig. 3).
The age at the peak of mortality has shifted to older ages in the more recent period, which is associated with birth cohort effect. After adjusting for birth cohort effect, mortality risk showed a sharp increase up to 60 years old, increased moderately during in the sixties, and subsequently increased again after 70 years old. This figure with an inflection in the sixties is similar to the age effect of breast cancer mortality reported from Osaka Japan [11] and Andalucia, Spain [12]. This phenomenon has been named Clemmensen’s Hook, which is the overlapping of two curves corresponding to preand post-menopausal tumors [13]. Some researchers argue that Clemmensen’s hook has disappeared [14]. A recent publication reported that Clemmensen’s hook might disappear in 2022, so that Clemmensen’s hook could be interpreted not as an overlapping of the two curves corresponding to preand post-menopausal tumors, but as a reflection of a shift in lifestyle among different generations or cohorts [8]. As shown Fig. 3, the Clemmensen’s hook in our data still exists until the year 2020 even though the shape of the hook has changed. Without a new birth cohort we are not certain that Clemmensen’s hook would disappear in the future in Korea.
The increased risk of breast cancer mortality among women born before 1968 may be mainly derived from the increased incidence rate of breast cancer, which is linked to an increase in prevalence of breast cancer risk factors, especially in reproductive factors and diet [15]. Epidemiologic studies conducted among Korean women have identified reproductive factors associated with breast cancer risk [4,16]. Early menarche, late menopause, a late full-term delivery, and never having breast-fed a child associated with the cohort effect of breast cancer incidence among Korean women. According to statistics from the National Statistical Office in Korea, total fertility rate per fertile Korean woman fell from 4.53 children in 1968 [17] to 1.30 in 2012 [2]. Age at first marriage increased from 21.6 years in 1960 to 29.6 years in 2013 [2], and age at first full-term delivery is increasing every year. The proportion of women breast-feeding their child showed a reduction from 59% in 1985 to 24.2% in 2006 [2]. The proportion of calorie intake from animal-based foods doubled during 1965-1995; on the other hand, the proportion of calorie intake from plant-based foods decreased from 97% in 1965 to 79% in 1995 [18]. Higher consumption of a Western-style diet is associated with earlier onset of menarche and earlier manifestation of hyperinsulinemic insulin resistance [19,20]. Postmenopausal obesity is also suggested as a risk factor for breast cancer in Korea [3,4], and the proportion of postmenopausal obese women is increasing [18].
Female breast cancer mortality risk decreased among women born after 1969 in Korea. Since the breast cancer incidence rate has shown a steady increase, the decreasing risk of mortality might be associated with a prolonged survival benefit: the 5-year relative survival rate in the 15-29 age group improved from 67.9% in 1993-1995 to 86.6% in 2006-2010 among Korean women [21]. Even though the survival benefit from better treatment in younger women may have caused that decreasing risk after the 1969 cohort, the wide confidence intervals due to the small number of breast cancer deaths shown in (a) of Fig. 2B suggest caution in interpretation of this result. Furthermore, since the annual increase in percent changes of incidence (from 1993 to 2002) and mortality (from around 1990 to 2006) are both the largest in Korea compared with those in other Asian countries/cities including Japan, China, Singapore, and Taiwan [9,22], further encouragement is needed for augmentation of breast cancer prevention and improvement of survival of breast cancer in Korean women.
In the current study the findings of an increased risk of breast cancer mortality among women born before 1968 then a decreased risk among women born after 1969 are consistent with previous results among Japanese women. In the age-period-cohort analysis of female breast cancer in Osaka between 1968 and 2007, breast cancer mortality risk increased among women born until the 1950s, and decreased thereafter [11]. It is considered that the changes in breast cancer risk factors in Asian countries can be largely attributed to rapid economic development. Economic development in Korea occurred after the end of the Korean War in 1953, which was more recent and rapid than the development in Japan. These year gaps in the boost of economic development are linked to the time gap of changes in risk factors between the two countries, which might have been reflected in the difference in birth years at the peak of mortality risk from the age-period-cohort models.
Mortality is a parameter resulting from various factors, including risks of disease, early detection rate, disease survival, and progress of surgical, radiological, and adjuvant systemic therapies for the disease; therefore, interpretation of breast cancer mortality trends is challenging [15]. In Korea, since 1999 the National Cancer Screening Program has recommended that women older than 40 years undergo a biennial mammographic examination, which in 1999 applied to generations born before 1959. Free screening services were expanded to all National Health Insurance beneficiaries in 2002-2003. Breast cancer screening rates were the highest among women in their fifties and the lowest among women over 70 years old [23,24]. The breast cancer screening rate, defined as the proportion of respondents who fulfilled the screening recommendation criteria, increased from 33.2% in 2004 to 70.9% in 2012, and the annual percent change was 4.5% (95% CI, 3.9% to 5.1%) [24]. Even though the coverage rate has shown a sharp increase, the short history of breast cancer screening makes it impossible to observe the impact of breast cancer screening in the short period of mortality data.
Conclusion
In summary, in this study the mortality pattern of breast cancer in Korea can be predominantly explained by a birth cohort effect, although age, period, and its combined effects affect the increasing trend of mortality. An increasing risk of breast cancer mortality was observed among women born before 1968, and a decreasing risk was observed among women born after 1969. Hence, the overall mortality of breast cancer may increase in Korea for a while, and show a gradual decrease in the future starting from the younger age group.
Notes
Conflict of interest relevant to this article was not reported.