Novel Germline Mutation of BRCA1 Gene in a 56-Year-Old Woman with Breast Cancer, Ovarian Cancer, and Diffuse Large B-Cell Lymphoma
Article information
Abstract
We report a case of a 56-year-old woman with breast cancer, ovarian cancer, and diffuse large B-cell lymphoma with a BRCA1 gene mutation. Evidence is mounting that there is a large increase in the risk for hematologic malignancies among patients with genetic changes in the BRCA pathways. The genomic analysis demonstrated a frameshift mutation in the BRCA1 gene: 277_279delinsCC (Phe93fs). It is a novel BRCA1 mutation that has never been reported, and caused malignant lymphoma as well as breast and ovarian cancer.
Introduction
Breast cancer is the second most common cancer in Korean women and is expected to continually increase [1]. Hereditary breast cancer accounts for 5%-10% of total cases. Most hereditary breast cancers are associated with mutations in the BRCA1 and BRCA2 genes [2], which confer high susceptibility to familial breast and/or ovarian cancer [3,4].
Associated with hereditary breast/ovarian cancer, the BRCA1 is the most extensively studied cancer susceptibility gene. Germline mutations of BRCA1 gene result in a predisposition for developing early-onset breast and ovarian cancer with penetrance as high as 85% and 65%, respectively [5]. The protein products of the BRCA1 gene regulate, at least in part, transcriptional activation, DNA repair, cell-cycle checkpoint control and chromosomal remodeling [6]. About 1,500 genetic variants of BRCA1 are described in the Breast Cancer Information Core (BIC). A recent meta-analysis reported a large increase in the risk for hematologic malignancies among patients with the genetic changes in BRCA pathways [7].
We report a case of a 56-year-old woman with breast cancer, ovarian cancer and diffuse large B-cell lymphoma with a novel BRCA1 gene mutation that has never been reported.
Case Report
A 56-year-old woman was referred to Korea University Anam Hospital for further management for recurred ovarian cancer. The patient was diagnosed with invasive ductal carcinoma in the left breast in 2005 (Fig. 1A). She had undergone modified radical mastectomy and axillary lymph node dissection. A right adrenal mass was incidentally found during staging work up (Fig. 2A), and a right adrenalectomy was done simultaneously with the mastectomy.

Radiologic and histologic features of the tumors. (A) The medial lateral oblique mammogram of the left breast shows 2×1.7-cm-sized oval shaped isodense mass with partially obscured margin at upper outer quadrant and 3.4×2.3-cm-sized enlarged axillary lymph node with loss of radiolucent fatty hilum at left axilla. (B) Invasive ductal carcinoma of breast (nuclear grade 2, histologic grade 2) reveals irregular infiltrative nests of tumor cells (H&E staining, ×200).
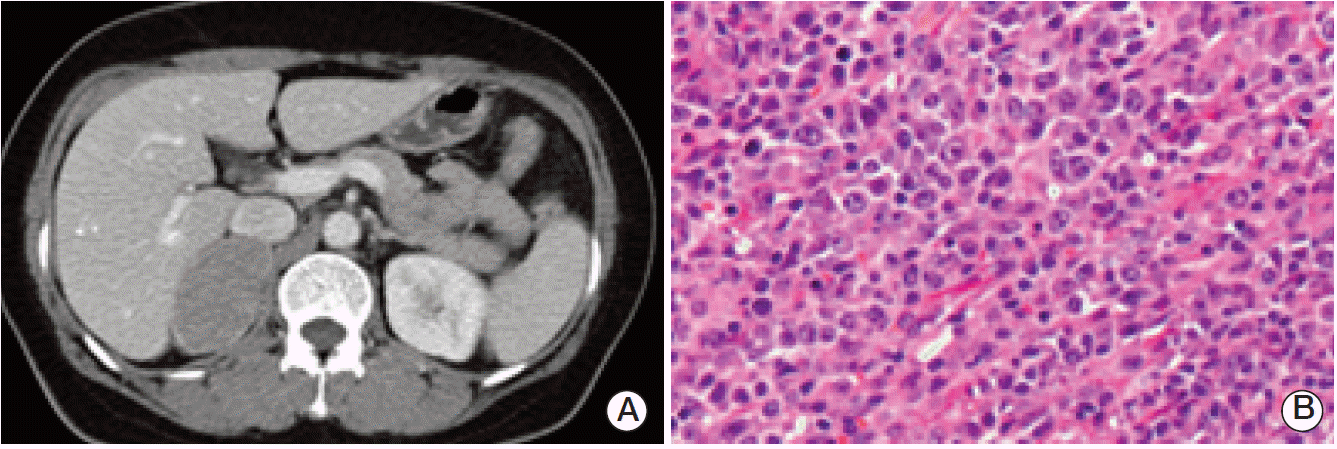
Abdomen computed tomography shows well defined homogeneously hypodense 5.4×3.7-cm-sized mass in right adrenal gland (A), and diffuse large B-cell lymphoma of adrenal gland shows diffuse proliferation of large atypical lymphoid cells with vesicular nuclei and multiple prominent nucleoli (B) (H&E staining, ×400).
Breast tumor pathology confirmed stage IIB invasive ductal carcinoma (T2pN1M0) (Fig. 1B), and the tumor was negative for estrogen receptor, positive for progesterone receptor, and negative for HER-2 overexpression. The 5.4×3.7-cm-sized adrenal mass was confirmed as diffuse large B-cell lymphoma (stage IA) (Fig. 2B). After the operation, she received chemotherapy with a CHOP regimen for four cycles. Adjuvant radiotherapy was applied to the left breast, left chest wall, and right adrenalectomy site. The patient continued tamoxifen from 2006 to 2010, but was lost to follow-up.
In 2011, the patient visited the hospital with abdominal discomfort. Abdominal computed tomography scan showed massive ascites with peritoneal thickening (Fig. 3A). Cytologic analysis for ascitic fluid revealed clusters of adenocarcinoma cells. Thus, she underwent both salpingooophorectomy, and pathology confirmed poorly differentiated, bilateral ovarian papillary adenocarcinoma (stage IV) (Fig. 3B). She received chemotherapy with docetaxel (60 mg/m2) and carboplatin (area under the curve, 6) every three weeks, and achieved a complete remission after six cycles of treatment. However, about six months later, she was referred to Korea University Anam Hospital for the management of recurring ovarian cancer. With the second line chemotherapy using gemcitabine and carboplatin, she is in complete remission as of July 2012.
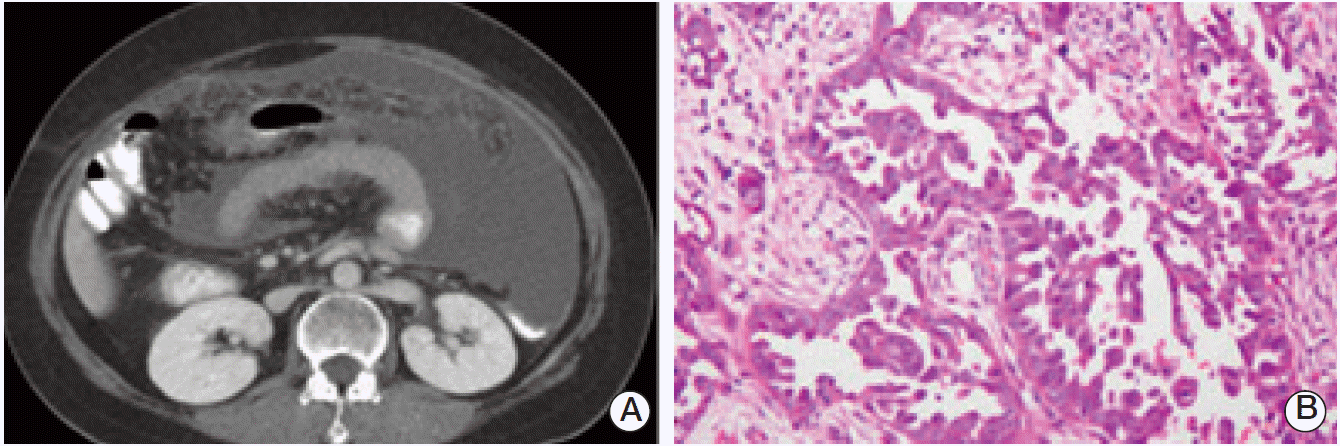
Abdomen computed tomography shows massive ascites with peritoneal thickening (A) and serous carcinoma of ovary reveals infiltrative tumor nests with papillary features (B) (H&E staining, ×200).
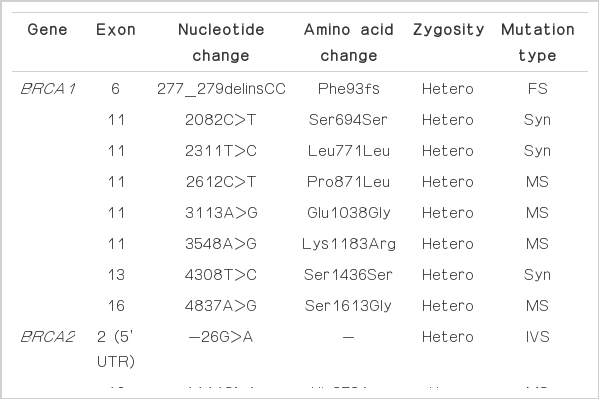
Since the patient revealed that her younger sister was also diagnosed with breast cancer at age 40, genomic analysis for BRCA1 and BRCA2mutations were performed (Table 1). The genomic analysis demonstrated a frameshift mutation in the BRCA1 gene: 277_279delinsCC (Phe93fs), a novel BRCA1 mutation never reported (Fig. 4). There was no mutation detected for the BRCA2 gene. Genomic analyses for other family members were also recommended.
Discussion
The BRCA1 mutation plays a significant role in breast and ovarian cancers. Most previous studies on BRCA1 were carried out in women of European ethnicities. Because of the increasing incidence and early age onset of breast cancer in Asian women, studies to determine the prevalence and penetrance of BRCA1 and BRCA2 mutation in Asian populations are under way in many Asian countries including Korea [8-10].
A study of 21 Korean hereditary breast/ovarian cancer families identified five deleterious mutations in the BRCA1 gene; two frameshift and three non-sense mutations, without polymorphisms or unclassified variants [10]. Frameshift mutations were 4159delGA and 5221delTG, which produced a truncated protein signal at codons 1354 and 1714. Three non-sense mutations were 3459G>T (E1114X), 4014C>T (Q1299X), and 5563G>A (W1815X).
An interim report of the Korean Hereditary Breast Cancer (KOHBRA) study was recently published [11,12]. The investigators of the KOHBRA study prospectively estimated the prevalence of BRCA mutations among Korean breast cancer patients with high risk for genetic changes. Among 853 probands, 167 mutation (19.6%) carriers were identified. The prevalence of the BRCA mutation was 24.8% for breast cancer patients with a familial history of breast/ovarian cancer and 11.3% for patients with early-onset (< 35 years) breast cancer without a familial history. They identified 33 types of BRCA1 mutations in 68 cases. The most frequent mutations in BRCA1 gene were 509C>A and 5615_5625del11insA, where each was found 11 times.
In our case, a frameshift mutation in the BRCA1 gene: 277_279delinsCC (Phe93fs) was noted. This novel BRCA1 mutation is not reported in the literature. The patient’s breast cancer and subsequent ovarian cancer and her sister’s early breast cancer might be affected by this genetic change. This novel BRCA1 mutation is thought to be related to hereditary breast/ovarian cancer. Of note, our patient exhibited simultaneous diffuse large B-cell lymphoma when she was diagnosed with breast cancer. Unlike the relationship between BRCA1 mutation and breast or ovarian cancers, the role of BRCA in the pathogenesis of malignant lymphoma has not been established. However, there are several studies supporting the hypothesis that BRCA1 mutation affects the development of hematologic malignancies through defective DNA repair pathways. In a meta-analysis [7], there was a large increase in the risk of hematologic malignancies in patients with mutant genes in the BRCA pathway; mantle cell lymphoma, acute myeloid leukemia, acute lymphocytic leukemia, chronic lymphocytic leukemia, and prolymphocytic leukemia. BRCA1 deficiency was strongly associated with both de novo and therapy related acute myeloid leukemia in the study. Some epidemiologic studies [13,14] also found increased risks for leukemia/lymphoma in identified BRCA1 or BRCA2 mutation carriers. Moreover, recent study on polymorphisms in DNA repair pathways reported increased risk of diffuse large B-cell lymphoma in BRCA1 mutant patients [15].
These data suggest that a family history of hematologic malignancies, in addition to breast/ovarian cancers, could be used to determine eligibility for mutation testing of BRCA1/BRCA2 in breast cancer patients. The significance of BRCA1 mutation in the pathogenesis of malignant lymphoma and other hematologic malignancies should be further investigated.
Notes
Conflict of interest relevant to this article was not reported.