Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy
Article information
Abstract
Purpose
Tumor response to neoadjuvant chemotherapy (NAC) may adversely affect the identification and accuracy rate of sentinel lymph node biopsy (SLNB). This study was conducted to evaluate the feasibility of SLNB in node-positive breast cancer patients with negative axillary conversion after NAC.
Materials and Methods
Ninety-six patients with positive nodes at presentation were prospectively enrolled. 18Fluorodeoxyglucose-positron emission tomography (18F-FDG PET) and ultrasonography were performed before and after NAC. A metastatic axillary lymph node was defined as positive if it was positive upon both 18F-FDG PET and ultrasonography, while it was considered negative if it was negative upon both 18F-FDG PET and ultrasonography.
Results
After NAC, 55 cases (57.3%) became clinically node-negative, while 41 cases (42.7%) remained node-positive. In the entire cohort, the sentinel lymph node (SLN) identification and false-negative rates were 84.3% (81/96) and 18.4% (9/49), respectively. In the negative axillary conversion group, the results of SLNB showed an 85.7% (48/55) identification rate and 16.7% (4/24) false-negative rate.
Conclusion
For breast cancer patients with clinically positive nodes at presentation, it is difficult to conclude whether the SLN accurately represents the metastatic status of all axillary lymph nodes, even after clinically negative node conversion following NAC.
Introduction
Sentinel lymph node biopsy (SLNB) is now a standard technique that has replaced axillary lymph node dissection (ALND) for axillary staging in early breast cancer patients that has resulted in much lower morbidity [1]. A sentinel lymph node (SLN) identification rate of 97.2%, accuracy rate of 97.1%, and false-negative rate (FNR) of 9.8% were reported in a large multi-institutional randomized study [2]. If the SLN is free of tumors following SLNB, the probability of cancer cells in the remaining axillary nodes is assumed to be less than 10%, in which case completion of ALND can be omitted [3]. SLNB is currently recognized as a suitable replacement for axillary dissection for staging procedures in clinically node-negative T1 and T2 breast carcinomas [4].
Over the past decade, neoadjuvant chemotherapy (NAC) has become increasingly common for the treatment of locally advanced breast cancer [5]. However, the role of SLNB in patients receiving NAC remains controversial. Nevertheless, meta-analysis for evaluation of the feasibility of SLNB after NAC suggested that SLNB is a reliable tool for planning treatment after preoperative chemotherapy [6]. Although other small, single-institution investigations of the efficacy of SLNB after NAC varied widely in identification rate and FNR [7,8], recent analysis of larger and multicenter data sets [9] revealed that SLNB after NAC seems to have a similar performance outcome as SLNB before systemic therapy [10]. Additionally, a National Cancer Institute conference recently reported that SLNB could be performed after NAC in patients with clinically negative nodes at initial diagnosis [11].
However, it is still unclear if the inclusion of patients with negative nodes at presentation for whom the results of SLNB may not be affected by NAC is inappropriate for accurate estimation of the predictive value of SLNB after NAC. Many previous studies of SLNB after NAC included patients with clinically negative nodes at presentation [12]; accordingly, the FNR calculated in those studies may have been underestimated, resulting in the outcome being recognized as acceptable when compared with SLNB before chemotherapy. As a result, other investigators have launched trials that exclusively enrolled patients with clinically positive nodes to evaluate SLNB after chemotherapy [13-17].
Nevertheless, the argument against SLNB following NAC has been made for patients with clinically positive nodes. As shown in Fig. 1, the response to systemic therapy can differ in each metastatic node, regardless of whether it is an SLN or non-SLN. For these patients, the theory of SLNs, which is postulated to be the first lymph node reached by metastasizing cancer cells from the tumor, could not be equally fitted.
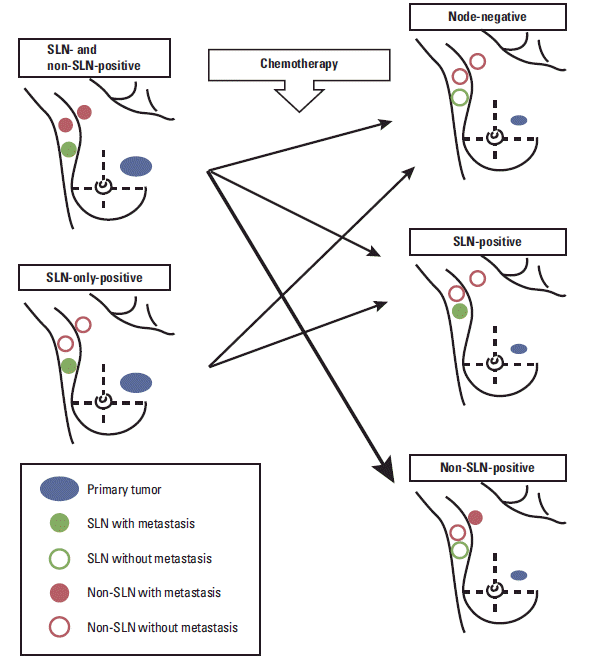
Various treatment outcomes according to axillary node status reflect an uneven tumor response to chemotherapy by each metastatic lymph node. Residual non-sentinel node metastasis after chemotherapy could raise concerns regarding application of sentinel lymph node (SLN) biopsy for patients receiving neoadjuvant chemo-therapy (red arrow).
In this study, we investigated node-positive breast cancer patients with negative axillary conversion after NAC. Patients with negative node conversion following NAC were placed in the same context as clinically node-negative patients. The primary goal of this study was to evaluate the feasibility of SLNB in node-positive breast cancer patients with negative axillary conversion after NAC in a prospective clinical trial.
Materials and Methods
1. Patients and protocol
From January 2007 to December 2009, 96 patients with T1-3 and documented axillary involvement at diagnosis were prospectively enrolled at Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. An overview of the study is provided in Fig. 2. Only patients with axillary involvement upon initial presentation were registered for this study.
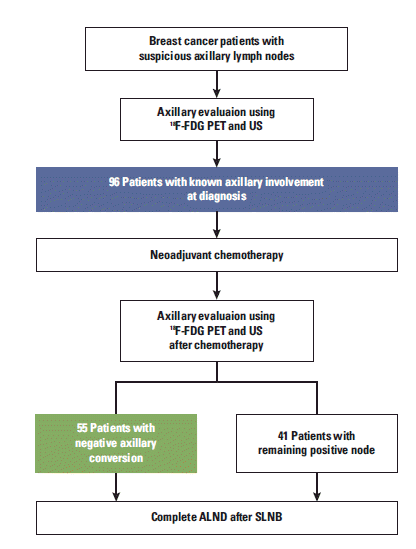
Study overview. 18F-FDG PET, 18Fluorodeoxyglucose-positron emission tomography; US, ultrasonography; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy.
In this study, an accurate evaluation of the axillary lymph node is crucial. In a previous report, we proposed that combined evaluation of ultrasonography (US) and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) could increase the accuracy of axillary staging [18]. Evaluation of axillary nodal status using both modalities was performed before and after chemotherapy in the present study. US scans of axillary lymph nodes were evaluated for shape, cortical thickening, and morphology of the hilum. A finding was considered positive if 1) the cortex of the nodes was concentrically or eccentrically thickened by more than 3 mm; 2) the node showed compression of the hilum and absence of the fatty hilum; or 3) a length-to-width ratio less than 1.5 was observed. If the axillary lymph nodes exhibited any of these characteristics, they were defined as suspicious for axillary lymph node metastasis. Nodes that exhibited none of the three characteristics mentioned above were defined as negative for axillary lymph node metastasis. Upon evaluation of 18F-FDG PET images, if the quantitative measurement of the single-pixel maximal standardized uptake value (SUVmax) was ≥ 2.0 in the ipsilateral axillary lymph node-bearing area it was considered positive for lymph node metastasis.
Nodes were considered clinically positive if the results of axillary evaluation showed positive results upon both US and 18F-FDG PET, regardless of palpable lymphadenopathy. After NAC, the patients were re-evaluated using these imaging studies. Patients with negative results following both exams were defined as the negative axillary conversion group. Patients with a positive node in any exam were defined as the remaining positive group.
All patients underwent complete ALND after SLNB for the axillary operation. Patients diagnosed with inflammatory breast cancer, T4, or who received palliative chemotherapy due to metastatic breast cancer were excluded. This study was prospectively initiated after approval from local institutional review boards. Informed consent was obtained from each participant in this study. This trial is registered with ClinicalTrials.gov number, NCT01622478.
2. Axillary lymph node mapping method
We conducted lymphatic mapping using technetium-99m (99mTc) tin colloid. Intradermal injection of 0.4 mL 30 MBq (0.8 mCi) 99mTc tin colloid diluted in normal saline solution was performed in 3-4 subareolar and intradermal areas. SLNs were determined by employing a gamma camera in the operating room (Gamma Detection System, Neoprobe Corporation, Dublin, OH). The node showing the highest radioactivity was dissected, after which the gamma detector was used again to confirm the correct node. All radioactive nodes with a count equal to or greater than 10% of the highest radioactive node were removed. If nodes could not be identified by the gamma probe, we proceeded with ALND.
3. Pathological evaluation of SLN
SLNs larger than 0.5 cm in the maximal dimension were serially sectioned transversely at 2-mm intervals, while those smaller than 0.5 cm were bisected. The dissected lymph nodes were measured and frozen, after which 4-μm serial sections were prepared from a portion of the dissected lymph node. Following pathologic evaluation of the sections, the remaining tissue was fixed in 10% formalin, embedded in paraffin blocks, and subjected to hematoxylin and eosin staining. If the hematoxylin and eosin sections were negative for malignancy, other sections were cut and immunohistochemically stained for keratin using monoclonal anti-human cytokeratin (clone AE1/AE3, Dako, Carpinetria, CA). However, we did not consider cytokeratin-only SLN as metastatic nodes because the clinical significance of micrometastases detected by immunohistochemical examination is unclear [19].
4. Statistical analysis
The primary measurement was FNR in the negative axillary conversion group. Baseline characteristics were statistically analyzed with χ2 or Fisher exact tests. The nonparametric Mann-Whitney U test was used to compare the number of resected SLNs. We compared the identification rate, FNR, negative predictive value (NPV), and accuracy rate between the two groups using the χ2 test. A 2×2 contingency table was constructed to evaluate the feasibility of SLNB in the negative axillary conversion group. p-values were two-tailed, and values < 0.05 were considered to be statistically significant. Analyses were conducted using SPSS ver. 16.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
There were 55 patients in the negative axillary conversion group and 41 patients in the remaining positive group. Mortality did not occur during chemotherapy. Pathologic complete response (pCR) was defined as no evidence of residual invasive cancer, both in the breast and axilla. The rate of pCR in all patients was 14.6% (14 of 96).
The baseline characteristics of the two groups are summarized in Table 1. No significant differences between groups were observed prior to NAC. Similarly, except for the proportion of surgical methods, no significant differences were found in characteristics related to surgical outcome including pathologic tumor size, rate of pCR, estrogen receptor status, progesterone receptor status, and human epidermal growth receptor-2 status. The proportion of breast conservation surgery was higher in the negative axillary conversion group (20.0% vs. 4.8%, p=0.035).
2. Results of SLNB
The results of SLNB for all patients and the two groups are listed in Table 2. For all patients, the median number of identified SLNs was 2 (range, 1 to 7), and lymphatic mapping was successful in 81 patients (84.3%, 81 of 96). FNR, NPV, and the accuracy rate were calculated to be 18.4% (9 of 49), 78.0% (32 of 41), and 87.9% (72 of 81), respectively.
The negative axillary conversion group showed an identification rate of 85.7% (48 of 56), a FNR of 16.7% (4 of 24), a NPV of 85.7% (24 of 28), and an accuracy rate of 91.7% (44 of 48). There was no significant difference in SLNB values between subgroups. A 2×2 contingency table for the negative axillary conversion group was constructed to evaluate the feasibility of SLNB (Table 3). The parameters of SLNB are detailed in the footnotes to Table 3.
The characteristics of the four false-negative patients in the negative axillary conversion group are presented in Table 4. All patients underwent mastectomy. Two patients received chemotherapy consisting of the adriamycin-docetaxel regimen, while the others received the adriamycin-cyclophosphamide regimen. The actual number of metastatic lymph nodes was one in all patients, but the maximum dimension of metastatic focus in non-SLN was larger than 2 mm, indicating macrometastasis.
Discussion
When studies to evaluate the feasibility of SLNB following NAC were designed, there was disagreement regarding whether patients with clinically negative nodes should be included. The results of SLNB may not be affected by chemotherapy in patients with negative nodes at presentation. Therefore, it is necessary to conduct studies of patient that had clinically positive nodes before NAC to evaluate SLNB after chemotherapy. Previous studies of SLNB after NAC for patients with clinically positive nodes at presentation reported identification rates of 77.6% to 98.0% and FNRs of 5.6% to 25% [13-15]. Our findings of these parameters for all patients showed results in the mid-range of these previous numbers, with an SLN identification rate of 84.3% and an FNR of 18.4, which are too high to justify use of SLNB after NAC. Otherwise, these studies included patients with clinically positive nodes after NAC, in whom SLNB are not indicated by the current treatment guideline [20]. Therefore, a systematic protocol for re-evaluation of the axillary nodal status following NAC is warranted.
Based on procedures commonly used in daily practice, we defined positive nodes using only imaging modalities because fine needle aspiration biopsy for axillary staging is not routinely recommended during diagnostic work-ups. To improve the accuracy of axillary staging without pathological confirmation, we used combined imaging modalities of 18F-FDG PET and US. Gil-Rendo et al. [21] reported that 18F-FDG PET could be used to assess axillary status accurately with a positive predictive value of 98.4% in another prospective study.
Another background characteristic of this study is the uneven response to chemotherapy by each metastatic node. As shown in Fig. 1, the tumor response to chemotherapy may vary independently in each metastatic node. Therefore, persistence of residual disease or undetected metastases in non-SLNs may not be predicted by a negative finding from SLNB, even in patients with a clinically negative axillary status after systemic therapy. The uneven response to chemotherapy is an important reason that many surgeons are currently unable to establish a role for SLNB in patients receiving NAC.
The use of SLNB is well established for patients with clinically negative nodes upon initial diagnosis, and its use contributes to axillary staging, expanding the opportunity to spare the patient from ALND for early breast cancer. Several reports have suggested that an FNR of 2% to 5% would be reasonable to justify conducting SLNB [4,22]. Moreover, expert panels have recommended that SLNB can be used to replace formal axillary dissection in the majority of T1 and T2 patients with clinically node-negative breast cancer when surgeons consistently achieve a detection rate of 90% and an FNR of 5% for representation of the entire axillary lymph node status [4].
One important aspect that must be considered in clinical practice is the need to identify a certain group with negative axillary conversion after chemotherapy among patients with positive nodes upon initial presentation. It is worth identifying patients who are clinically node-positive after chemotherapy in whom SLNB are not indicated by the current treatment guidelines [20]. Therefore, we separated the patients according to a nodal response, and mainly analyzed the results of patients with negative node conversion. The identification rate of SLN and FNR was 85.7% and 16.7%, respectively, in these patients. This FNR was higher than the expert panel’s recommendation and reasonable values of FNR [4,22]. Knowing the exact pathologic staging acquired by conventional surgery would be more valuable for prognosis than other predictive models for patients following NAC. Recent meta-analysis and pooled analysis based on a neoadjuvant clinical trial suggested that pCR, defined as no residuals in nodes or breasts, can best discriminate between patients with favorable and unfavorable outcomes [23,24]. These findings support the importance of axillary staging for patients receiving NAC. Accordingly, the high FNR with SLNB reported in our study raises concerns with regard to the reliability of SLNB for patients with clinically negative nodes after NAC.
Two large clinical trials to determine the accuracy of SLNB in patients with clinically positive nodes were recently reported. Specifically, an American College of Surgeons Oncology Group Z1071 clinical trial (ACOSOG Z1071) reported that SLNB after NAC in node-positive breast cancer patients correctly identified nodal status in 84% of all patients and that FNR was 12.8% in patients of clinical N1 with more than two resected SLNs [16]. The key difference in their trial design and our study is that they omitted axillary reevaluation after NAC.
The results of the prospective German multi-institutional SENTINA-trial (SENTINA) are more concordant with our findings. In arm C of the SENTINA-trial, they enrolled patients with clinically negative axillary conversion after NAC based on the results of US. The rate of FNR in 592 patients was 14.2%. The investigators concluded that use of SLNB as a diagnostic procedure is not a reliable tool in patients who convert from clinical N1 to clinical N0 under NAC when compared to SLNB during primary surgery [17].
It should be noted that this study has several limitations. For example, there was a small number of enrolled patients; thus, the outcome was largely affected by a single event. Moreover, the definition of clinically negative node status was determined based on both 18F-FDG PET and US, which made it difficult to enroll patients to the negative axillary conversion group. Nevertheless, the results of this study have clinical significance and provide important insight into the validation of SLNB following NAC.
In future studies, subgroups of positive node breast cancer patients with negative axillary conversion who are safely eligible for SLNB should be identified. Resection of at least three SLNs and adoption of dual tracers that were suggested by the two prospective trials will be useful in identification of suitable subgroups.
Conclusion
Overall, the results of this study indicate that routine use of SLNB is not feasible for patients who undergo NAC, even after clinically negative node conversion following NAC.
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by a grant from the research fund of Yonsei University Medical College, Seoul, Korea (2008-0166).
The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for his help with the figures.



