Cytoplasmic Expression of HuR is Related to Cyclooxygenase-2 Expression in Colon Cancer
Article information
Abstract
Purpose
HuR, human family embryonic-lethal abnormal vision-like protein, can bind to mRNA and stabilizes the nucleic acid in the cytoplasm, resulting in more efficient translation. HuR is predominantly present in the nucleus and shuttles between the nucleus and cytoplasm. HuR stabilizes cyclooxygenase-2 (Cox-2) mRNA in several cancers, including breast, stomach, lung and brain cancer.
Materials and Methods
We investigated the expression and cellular location of HuR, as well as evaluated Cox-2 expression in 79 colorectal cancer patients with the use of immunohistochemical methods. The biological implications of HuR localization and Cox-2 expression in colorectal carcinoma were evaluated.
Results
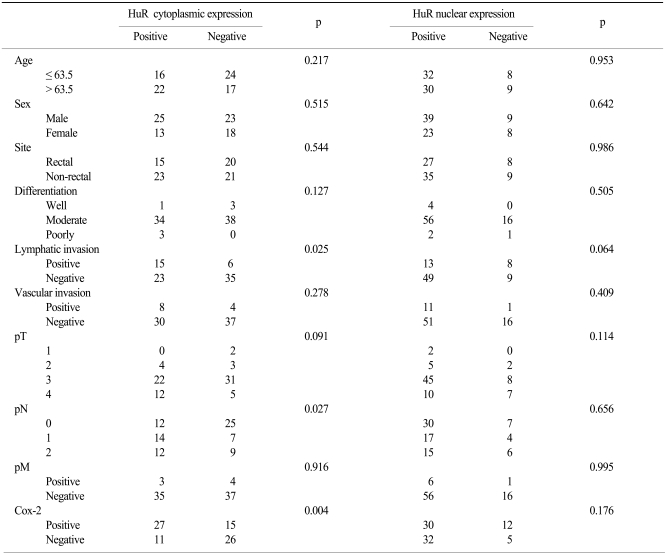
Nuclear HuR expression was observed in 59 (74.7%) tumors and cytoplasmic HuR expression was seen in 25 (31.6%) tumors. Cox-2 immunoreactivity was noted in 42 (53%) tumors. The expression of cytoplasmic HuR was significantly associated with Cox-2 expression (p=0.004). Cytoplasmic expression of HuR showed a correlation with lymphatic invasion (p=0.025) and the presence of a lymph node metastasis (p=0.027). The presence of nuclear HuR showed no correlation with Cox-2 expression or any other of the clinicopathological parameters that were examined.
Conclusion
These results suggest that cytoplasmic translocation of HuR is associated with Cox-2 expression for some colorectal carcinomas.
Introduction
The regulation of gene expression in eukaryotes is controlled at both the transcriptional and posttranscriptional level (1). Post-transcriptional gene regulatory events, especially regulation of mRNA turnover, have emerged as fundamental and effective means to alter the expression of functionally related genes (2). The investigation of post-transcriptional regulatory mechanisms centers on the association of a given RNA-binding protein with its target mRNA (3). The proteins make mRNA stable by binding to AU-rich elements (AREs) in the 3'-untranslated region of some mRNAs. Thus, the binding effectively lengthens the presence of a given mRNA and consequently, the amount of protein expressed is increased. Among the ARE-binding proteins identified, the human family embryonic lethal abnormal vision (ELAV)-like proteins that consist of four members (Hel-N1/HuB, HuC, HuD and HuR) have been well characterized (4). HuR is expressed in many cell types, mainly in the cellular nucleus, while the other three proteins are usually expressed in terminally differentiated tissue (4). Several studies have shown that HuR can shuttle between the nucleus and cytoplasm and localization of HuR in the cytoplasm is required for its mRNA stabilizing function (5). HuR binds to labile transcripts containing AREs, such as mRNAs for proto-oncogenes, cytokines and cytokine-response genes in the nucleus. The HuR-mRNA complex is then transported to the cytoplasm (6), where the stabilized mRNA can be translated into protein more efficiently.
Increased expression of cyclooxygense-2 (Cox-2) has been implicated in carcinogenesis. There is evidence that mRNA stability and translational efficiency are major steps for the control of Cox-2 expression, although an increase of Cox-2 gene transcription partly accounts for the increase of Cox-2 protein expression (7,8). There is an ARE in the 3-untranslated region of Cox-2 mRNA that provides the binding site for HuR, resulting in increased mRNA half-life (9). Cox-2 is overexpressed in several malignant tumors and the increase in expression may due to dysregulation of mRNA stability (10-12).
Recently, a correlation between the protein expression level of HuR and Cox-2 was found in colon, breast, stomach, ovary and uterine cervical cancer cells (9,13-16). There have been no reports on the relationship between HuR and Cox-2 expression in colorectal cancer tumors from patients in South Korea.
Colorectal cancer is one of the leading causes of cancer-associated death and the third most common malignant tumor found in patients in Western countries (17). The incidence and the mortality rate of colorectal cancer have also been on the increase in South Korea (18). We investigated the expression and cellular location of HuR and measured Cox-2 expression with the use of immunohistochemical methods. The biological implications of HuR localization and Cox-2 expression in colorectal carcinoma were also evaluated.
Materials and Methods
We collected 79 cases of primary colorectal cancer patients from May 2006 to December 2007 at the East-West Neo Medical Center. The clinical characteristics of patients were evaluated from hospital records. Resected tissue specimens were fixed in 10% buffered formalin and were embedded in paraffin. Histological sections cut from paraffin blocks were stained with hematoxylin and eosin, and all of the stained slides were reviewed. For effective and fast detection, tissue array slides were made. Two cores of representative cancer tissue (2.0 mm in diameter) were obtained from the original paraffin block and the cores were inserted into a new paraffin block with 40 holes. The tissue microarray block also contained three normal colorectal cores. Serial sectioned slides were produced and hematoxylin and eosin staining was performed.
Immunohistochemical procedures were carried out on 4-µm tissue sections from each tissue microarray block using the Bond Polymer Intense Detection system (Leica Microsystems, North Ryde, NSW Australia) according to the manufacturer's instruction with minor modifications. In brief, 4-µm sections of formalin-fixed and paraffin-embedded tissues were deparaffinized by the use of Bond Dewax Solution (Leica Microsystems), and an antigen retrieval procedure was preformed using Bond ER solution (Leica Microsystems) for 30 minutes at 100℃. Endogenous peroxidase was quenched by incubation with hydrogen peroxide for five minutes. Sections were incubated for 15 minutes at ambient temperature with a monoclonal COX-2 antibody (1 : 400 dilution; Dako, Glostrup, Denmark) and a monoclonal HuR antibody (3A2, 1 : 300 dilution; Zymed, South San Francisco, CA), respectively, using biotin-free polymeric horseradish peroxidase (HRP)-linker antibody conjugate system in a Bond-max automatic slide stainer (Leica Microsystems). As a positive control for Cox-2 expression, we used a human colon carcinoma cell line known to overexpress Cox-2. When more than 10% of the cancer cells showed cytoplasmic staining for Cox-2, the tumor was judged as positive for Cox-2 expression. We observed normal breast glands as a positive control for HuR staining. We evaluated the percentage of positive cells and intensity of staining for the evaluation of the HuR staining status. The percentage of positive cells was scored as follows: 0 (0% positive cells), 1 (< 10% positive cells), 2 (10 to < 50% positive cells), 3 (50 to < 80% positive cells), 4 (> 80% positive cells). The intensity of cytoplasmic and nuclear staining was scored as follows: 0 (negative staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining). For the immunoreactive score, values from 0 to 12 were multiplied by the percentage of the positive cell score and intensity of the staining score. We classified the cases as having negative or weak expression when the immunoreactive score was 0 to 5; a score from 6 to 12 was regarded as strong expression.
All statistical analyses were performed using MedCalc 9.6.0.1 statistical software. The relationships between variables were studied using the chi-squared test. A probability value below 0.05 was considered as statistically significant.
Results
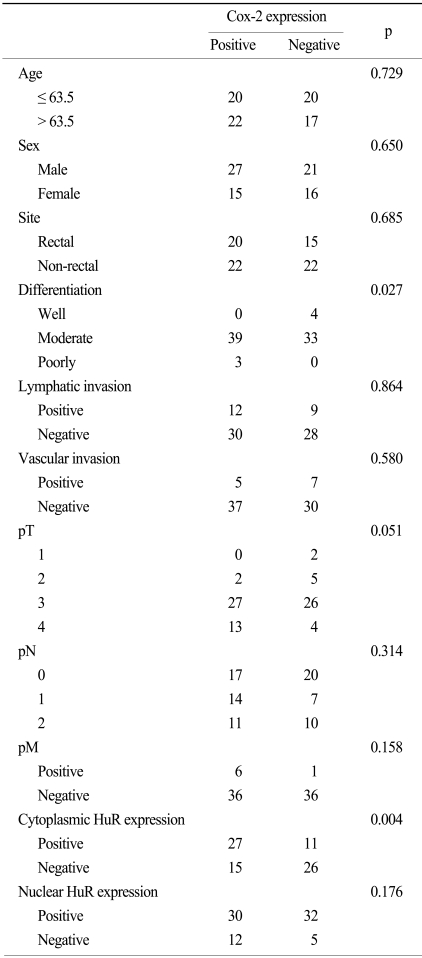
The mean and median age of the patients at the time of the initial diagnosis was 62.4 years (age range, 39~85 years) and 63.5 years, respectively. Of all of the patients, 48 (60.8%) were male. In the study population, 67.1% of patients had moderately differentiated tumors, 5.1% of the patents had well differentiated tumors and 3.8% of the patients had poorly differentiated tumors. Of the 79 patients, 35 patients (44.3%) had a rectal cancer while 44 (55.7%) patients had a non-rectal colon cancer. A lymph node metastasis was present in 42 patients (53.2%). Baseline patient characteristics are shown in Table 1. HuR expression in the nucleus and cytoplasm of cancer cells was present in 59 (74.7%) and 25 (31.6%) cases, respectively (Fig. 1A, B). As shown in Table 2, cytoplasmic expression of HuR significantly correlated with Cox-2 expression (p=0.004) and lymphatic invasion (p=0.027). Other clinicopathological factors such as age, sex, site, differentiation, vascular invasion and TNM stage showed no significant correlation with HuR cytoplasmic expression. For nuclear expression of HuR, we could not observe any association between nuclear expression of HuR and the clinicopathological factors (Table 2). Cox-2 expression was seen in 42 (53%) colorectal cancer cases (Fig. 2). The expression of Cox-2 in the adjacent normal colorectal mucosal epithelium was very weak or negative. Cox-2 expression showed a significant correlation with differentiation (p=0.027). Cox-2 expression increased with a higher T stage, but the finding was not statistically significant (p=0.051). Other clinicopathological factors examined showed no significant correlations (Table 3).
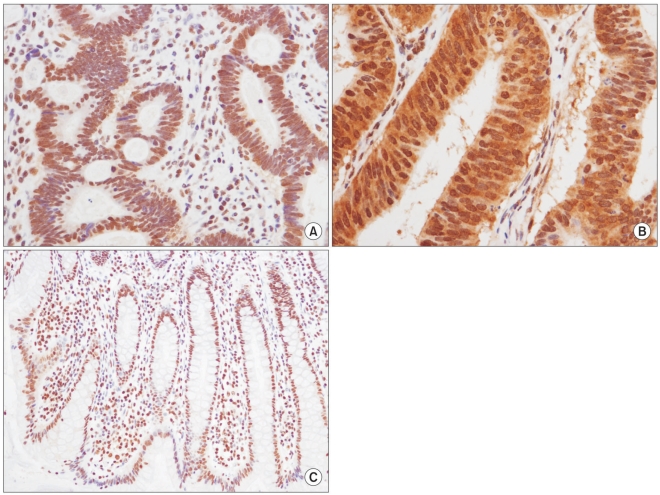
Strong nuclear (A,×400) and cytoplasmic expression (B,×400) of HuR were noted in a colorectal cancer specimen, but the normal colonic mucosa showed diffuse nuclear staining in the crypt glands (C,×200).
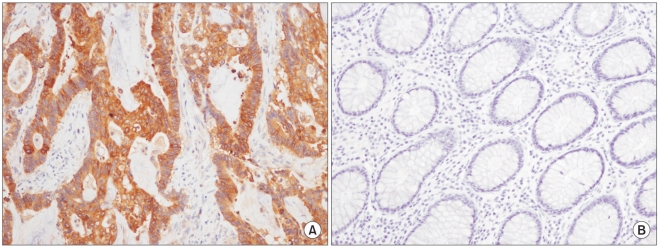
Diffuse strong cytoplasmic expression of Cox-2 for an adenocarcinoma in the colon (A,×200) was seen, and no staining was seen in the normal mucosa (B,×200).
Discussion
In this study, we demonstrated that colorectal cancer showed predominantly nuclear expression (74.7%) of HuR, and increased cytoplasmic expression of HuR was significantly associated with increased lymph node metastasis (p=0.027) and lymphatic invasion (p=0.025), whereas nuclear expression of HuR showed no association with the examined clinicopathological parameters. Furthermore, we found that cytoplasmic expression of HuR was significantly associated with Cox-2 expression. Our results are in accord with the previous studies that reported that the HuR protein translocates from the nucleus to the cytoplasm during tumorigenesis (13-16). Eleven cases of negative Cox-2 expression showed positive cytoplasmic HuR expression in our study. This finding suggests that the possibility of other additional regulatory mechanisms for Cox-2 expression exist. Even though the regulation of mRNA stability of Cox-2 is thought to be an important regulatory step, other mechanisms such as transcriptional control can also exist. Further large-scale studies are needed for the determination of the mechanism of regulation of Cox-2 expression and stabilization of Cox-2 mRNA in cancer cells.
Although the ability of HuR to shuttle from the nucleus to cytoplasm is important for mRNA stabilization, the exact mechanism is not known. Several mechanisms for controlling the cellular location have been studied. Recent studies have shown that mitogenic-activated protein kinase-2 increased cytoplasmic translocation of HuR and the stability of ARE-containing mRNAs (19). The translocation of HuR to the cytoplasm can be induced by stress caused by agents such as UV light, DNA damaging agents or T cell activation, while AMP-activated kinase can inhibit the translocation of HuR to the cytoplasm (20-22).
There are some other mRNAs, such as the mRNAs for tumor necrosis factor α, cyclin A, cyclin B1, MARCKS, uPA and uPA receptor that are stabilized by HuR (23-25). Based on the known functions of HuR, we believe that HuR might play an important role in cell cycle regulation, apoptosis, angiogenesis, inflammation, and tumor growth. Moreover, HuR can be a potential target for molecular tumor therapy with consideration of these multiple effects.
Conclusion
The cytoplasmic expression of HuR may be a part of a regulatory pathway (s) that controls the expression of Cox-2 in colorectal cancer. Additional studies with a larger number of specimens are required to determine if HuR might be a potential target for tumor therapy.
Notes
This work was supported by the KyungHee University Research Fund in 2006 (KHU-20061221).