Clinical Outcome of Relapsed or Refractory Burkitt Lymphoma and Mature B-Cell Lymphoblastic Leukemia in Children and Adolescents
Article information
Abstract
Purpose
Despite the rapid improvement in survival rate from Burkitt lymphoma and mature B-cell lymphoblastic leukemia (B-ALL) in children, a small subset of patients do not respond to first-line chemotherapy or experience relapse (RL). Herein, we report the clinical characteristics and outcomes of these patients.
Materials and Methods
RL or refractory Burkitt lymphoma and mature B-ALL in 125 patients diagnosed from 1990 to 2009 were retrospectively analyzed.
Results
Nineteen patients experienced RL or progressive disease (PD). Among them, 12 patients had PD or RL less than six months after initial treatment and seven had late RL. Seven patients achieved complete response (CR), 11 had PD, and one had no more therapy. Six patients who achieved CR survived without evidence of disease and four of them underwent high-dose chemotherapy (HDC) followed by stem cell transplantation (SCT). However, 11 patients who failed to obtain CR eventually died of their disease. Five-year overall survival (OS) was 31.6±10.7%. OS of patients with late RL was superior to that of patients with early RL (57.1±18.7%, vs. 16.7±10.8%, p=0.014). Achievement of CR after reinduction had significant OS (p < 0.001). OS for patients who were transplanted was superior (p < 0.01). In multivariate analysis, achievement of CR after reinduction chemotherapy showed an association with improved OS (p=0.05).
Conclusion
Late RL and chemotherapy-sensitive patients have the chance to achieve continuous CR using HDC/SCT, whereas patients who are refractory to retrieval therapy have poor prognosis. Therefore, novel salvage strategy is required for improvement of survival for this small set of patients.
Introduction
With the introduction of highly intensive and multiagent chemotherapy, the survival rate from Burkitt lymphoma has improved dramatically to > 90% [1-4]. In an international cooperative study, patients with central nervous system (CNS)-positive and/or mature B-cell acute lymphoblastic leukemia (B-ALL), who had far advanced disease, were shown to have a cure rate of 80-90% with the addition of high-dose methotrexate (total dose, 24 g/m2), high-dose cytarabine arabinoside (Ara-C; total dose, 25.5 g/m2), and etoposide (VP16; total dose, 2,500 mg/m2) [1,5]. However, patients with relapse or who are refractory to first-line treatment have poor prognosis [6-12], and there are few reports on the incidence and treatment of relapsed or refractory pediatric Burkitt lymphoma and mature B-ALL. In 2004, the Korean Society of Pediatric Hematology-Oncology retrospectively analyzed the incidence, pathologic subtypes, treatment strategies, and survival rate of children with malignant lymphoma. However, the incidence and outcome of relapsed or refractory disease according to pathologic subtypes were not reported. Here, we report the characteristics and clinical outcomes of relapsed or refractory pediatric Burkitt lymphoma and mature B-ALL.
Materials and Methods
A total of 131 patients diagnosed with Burkitt lymphoma and mature B-ALL at five institutions between January 1990 and December 2009 were included in the current study. Six patients, including three who were discharged against medical advice, one whose medical record was lost, and two who were lost during follow-up were excluded from the analysis. Medical records of 125 patients were retrospectively reviewed for comparison of survival outcome. This study was approved by the institutional review board at each university hospital.
1. Response criteria
International Working Group recommendations were used to define response after treatment [13]. Disease status was determined based on bone marrow (BM) biopsy, cerebrospinal fluid (CSF) examination, and imaging studies. Complete response (CR) was defined as disappearance of symptoms and lesions detected by computed tomography or magnetic resonance imaging at diagnosis and normalization of CSF and BM. Partial remission (PR) was any response less than CR in the absence of progressive disease (PD), while PD was an increase in size > 25% in the product of the two largest dimensions or increase in the numbers of CSF and/or BM blasts. Relapse was defined as appearance of new lesions, blasts in the CSF or excess of blasts in the BM after CR.
Relapses were classified as early and late, with cut-off duration of six months. Time to relapse was the duration between the start day of chemotherapy or radiotherapy and the diagnosis of relapse.
2. First-line chemotherapy protocols
Various chemotherapy protocols were chosen according to each institution's decision. The lymphoma malignancy B (LMB) protocol of the Society of French Pediatric Oncology (SFOP) was most commonly used: LMB 89 or 96 [14] protocols in eight patients, LMB 81 [15] in one, Children's Cancer Group (CCG) 106B [16] in six, daunomycin-cyclophosphamide, vincristine, methotrexate, and prednisolone (D-COMP) [17] in two, LSA2-L2 [18] in one, and Berlin-Frankfurt-Munster 90 [19] in one.
3. Statistical analysis
Event-free survival (EFS) from the date of diagnosis to the date of relapse/progress or death and overall survival (OS) from the date of diagnosis to the date of last follow-up were calculated using the Kaplan-Meier method. Heterogeneity of survival curves was compared using the log-rank method. Cox's proportional hazards regression was used to fit a model for variables found significant by univariate analysis. p < 0.05 was considered significant. SPSS ver. 13.0 (SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
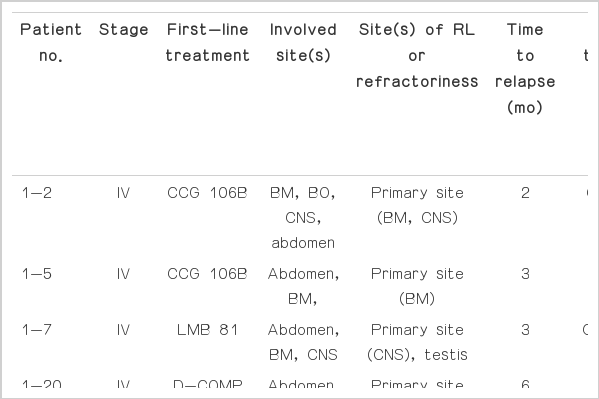
During the study, 19 of 125 patients (15%) experienced relapse or progression. The characteristics, treatment, and outcome of 19 patients are shown in Table 1. The median age of patients experiencing relapse or refractory disease at initial diagnosis was 8.8 years (range, 2.1 to 13.8 years) with a male predominance (17:2). Six patients initially presented with stage III and 13 presented with stage IV (including seven CNS-positive). Three patients had primary refractory disease and 16 patients experienced relapse. Median time to relapse was five months (range, 2 to 117 months). Sites of relapse or progress included the primary sites in 14 and new sites in five cases. Descending frequency was as follows: CNS (n=10), BM (n=8), abdomen (n=8), and lungs (n=2). The median lactate dehydrogenase level at diagnosis was 1,080 IU/L (range, 214 to 10,374 IU/L). Seven patients with available cytogenetic data had various cytogenetic abnormalities, including t(8;14)(q24;q32), except one (46, XY).
1. Response to first-line therapy
Three patients had progression, nine developed early relapse, and seven developed late relapse. Median survival for patients with PD or early relapse was three months (range, 0 to 130 months) and two are alive with CR. These two patients had isolated CNS relapse. Median time to relapse for late relapse was nine months (range, 6 to 117 months) and median survival had not been reached.
2. Salvage therapy after relapse or refractory disease
Two patients with PD received CCG 106B induction chemotherapy or rituximab but showed no response to reinduction chemotherapy and eventually died of disease within three months from the time of progression. Most frequently used reinduction chemotherapy in patients with relapse was CCG 106B induction used in five patients, two of whom achieved CR, one had PR, and two had PD. Other reinduction chemotherapy included high-dose cytarabine arabinoside, cyclophosphamide, oncovin, methotrexate chemotherapy used in three patients, CCG 1882B induction chemotherapy used in two patients, LMB 89 or 96 used in two patients, ifosfamide, carboplatin, etoposide (ICE) plus weekly rituximab used in one patient, and so on (see Table 1). As a response to reinduction chemotherapy seven patients achieved CR and three of them received high-dose chemotherapy (HDC) followed by autologous stem cell transplantation (ASCT), and drugs used in the HDC included bis-chloroethyl-nitrosourea or nimustin, etoposide, and cyclophosphamide in two patients and carboplatin and ifosfamide in one patient. One patient with mature B-ALL had fludarabine, cyclophosphamide and total body irradiation conditioned unrelated BM stem cell transplanatation (SCT). A patient (3-14) with multiple bone metastases who had already received ASCT in first-line treatment received eight cycles of ICE chemotherapy and 10 doses of rituximab (375 mg/m2/dose) after relapse and was alive without disease.
3. Outcome after salvage chemotherapy
Six patients (31.5%) survived, with a median follow-up period of 86 months (range, 71 to 134 months). Three patients with PD died of disease. However, seven patients who achieved CR after salvage therapy are alive, except one (nos. 1-55) without disease. One experienced second CNS relapse after maintaining short term remission. He received CCG 106B induction chemotherapy and radiotherapy with a dose of craniospinal irradiation (6 Gy/3Fr) and whole brain irradiation (18 Gy/9Fr) as a salvage therapy but eventually died of disease five months after relapse. Nine patients with PD after salvage therapy died of disease.
The OS and EFS of all 125 patients was 82.4±3.4% and 77.6±3.65%, respectively, whereas those of patients experiencing relapse or refractory disease was 31.6±10.7% (Fig. 1A). Significantly improved outcome (85.7±13.2%) was observed for patients who achieved CR to reinduction chemotherapy (p < 0.001).

(A) Overall survival of relapsed or primary refractory disease. Median survival was seven months (95% confidence interval [CI], 5.6 to 8.4). (B) Overall survival of patients who achieved complete response (CR) and non-CR to reinduction chemotherapy, which is statistically significant (p < 0.001). Median survival was 5.5 months (95% CI, 3.4 to 6.6) for patients who had non-CR. (C) Overall survival and time to relapse. Median survival was five months for patients who had primary refractory or early relapse. Survival rate for primary refractory or early relapse and late relapse was 16.7±10.8% and 57.1±18.7, respectively (p < 0.05). (D) Overall survival and hematopoietic stem cell transplantation. Median survival was 6.5 months (95% CI, 4.6 to 8.4) for patients who were not transplanted. Overall survival of both groups was statistically significant (p < 0.01).
The OS of early and late relapses was 16.7±10.8% and 57.1±18.7%, respectively (p=0.014) (Fig. 1C). The OS of those who were transplanted and were not transplanted were 100% and 13.3±8.8%, respectively (p=0.008) (Fig. 1D). To evaluate the impact of SCT on survival in patients who achieved CR after salvage therapy we compared the OS of patients who were transplanted (n=4) and those who were not transplanted (n=3), but there was no significance (p=0.25).
Multivariate Cox regression analysis using conditional parameter estimate showed that achievement of CR after reinduction chemotherapy was associated with improved OS (hazard ratio, 0.009; 95% confidence interval, 0.000 to 0.999; p=0.05) and other variables, including time to relapse and hematopoietic SCT had no influence on survival outcome.
Discussion
Despite dramatically improved survival over the past few decades, patients experiencing relapse or refractory disease continue to have very poor outcomes. The frequency of relapse vs. progress varies according to first-line treatment used; therefore, objective comparison is difficult. The incidence of relapse or refractory disease was reported as 6.4% (9 /140) in an Austrian multicenter study [12] and 10.1% (33/327) in a Japanese multicenter study [8], although the two groups used different treatment protocols. There are no data from a nationwide study. In our dataset, 15.2% (19/125) of patients with Burkitt lymphoma and mature B-ALL developed relapse or refractory disease. Diffuse large B cell lymphoma and primary mediastinal lymphoma were not included in our dataset and various first-line chemotherapy and reinduction chemotherapy were tried in multicenter during a long period (1990-2009).
Despite introduction of heterogenous reinduction chemotherapies, seven patients achieved CR (CR rate, 36.8%), six of whom are alive without evidence of disease. Another 12 patients who never responded to salvage regimens died with rapid disease progression. From this finding we can infer that responsiveness to retrieval therapy is a very important factor for survival of this small subset of patients (p=0.05, multivariate Cox's regression analysis).
In our cohort, five patients were assigned to the LMB risk group C (CNS positive in 3; CNS negative in 2) and received highly intensive chemotherapy at initial diagnosis (two cycles of COPADM as induction chemotherapy and CYVE as consolidation chemotherapy). After relapse, four patients received 106B reinduction chemotherapy. As a result, only one CNS-negative patient (nos. 1-64) responded to retrieval therapy and achieved CR.
Patte et al. [3] reported that among patients in LMB 84 group C (using high-dose methotrexate, high-dose Ara-C, and VP16), there were no survivors in patients whose tumors did not respond to the prephase chemotherapy, meaning that patients who failed to achieve remission with this intense first-line treatment receive virtually no benefit from HDC/SCT. Anoop et al. [20] recently reported that all 10 relapsed patients in group C (LMB 96 and UKCCSG2003) died despite receiving various reinduction treatments. Considering the high morbidity and mortality rates of LMB 89/96 protocols group C, we must refine the first-line treatments without compromising the current survival outcome. Cytogenetic and molecular data should be incorporated in stratification of patients and tailored treatment including rituximab and/or HDC/SCT is warranted in the near future.
Because a correlation between response to initial retrieval therapy and survival is evident, guaranteed salvage regimens should be chosen for treatment of relapse or refractory disease. Various retrieval therapies have been tried to this point. The DECAL regimen (dexamethasone, etoposide, cisplatin, cytarabine arabinoside, L-asparaginase) used by the CCG 5912 had a 50% response rate and a 30% 2-year OS that was independent of pathologic subtypes [21]. The ICE regimen (ifosfamide, carboplatin, and etoposide) used by the Pediatric Oncology Group had a 71% CR/PR rate, but long-term outcomes were not reported [10]. Another combination of rituximab plus ICE chemotherapy was used by the CCG and a 64% (9/14) CR/PR rate was reported. Responders to salvage treatment had significantly longer OS than patients who did not respond to treatment [21]. In addition, recently published data showed that rituximab (375 mg/m2/dose) is an important parameter affecting survival and four doses of rituximab (total dose 1,500 mg/m2) showed an association with improved survival in regression analysis. Responsiveness to retrieval therapy is a prerequisite for survival in patients experiencing relapse or refractory disease. Some benefits seem to be achieved by consolidative therapy using HDC/SCT in only a portion of patients who responded to salvage therapy [8,11,22].
If we can predict the risk of relapse or lack of response to chemotherapy at the beginning of first-line therapy, other treatment options such as HDC/SCT used with or without immunotherapy can be used at the time of initial diagnosis, which will be ideal for this dismal subset of patients. The importance of cytogenetic data was recently accentuated. More than 80% of Burkitt lymphoma and mature B-ALL patients are known to have the MYC/8q24 rearrangement. In addition to the MYC/8q24 rearrangement, other chromosomal aberrations have been described in 60-90% of Burkitt lymphoma [23]; these abnormalities are reported to be associated with tumor progression [24]. Association of cytogenetic abnormalities such as del(13q), +7q, complexity, and aneuploidy with a worse outcome has been reported [25], but a more comprehensive analysis of the correlation between chromosomal aberrations and clinical outcome with a larger number of patients is warranted for adjustment of cytogenetic risk-adapted therapy. Unfortunately, cytogenetic data in our patient set was not informative due to missing or unchecked data in a large portion of patients. In the future, cytogenetic data should be included as a basic component of initial diagnosis and also incorporated with treatment stratification.
Conclusion
Because efficient drugs have already been escalated to front-line therapy, new therapeutic approaches that are adjusted according to biological cancer cell behavior using cytogenetic and molecular data, such as ICE or CYVE chemotherapy plus rituximab and/or ASCT or allogeneic SCT, are needed for high-risk patients. Use of HDC/SCT might be beneficial for patients with relapsed or refractory disease who are responding to retrieval chemotherapy. Conduct of more observational study using a large cohort is warranted in order to estimate efficacy of these approaches.
Notes
Conflict of interest relevant to this article was not reported.
Acknowledgements
This work was supported by the fund of Research Promotion Program (RPP-2010-006), Gyeongsang National University.