Up-regulation of RhoGDI2 in Human Breast Cancer and Its Prognostic Implications
Article information
Abstract
Purpose
Recent research has identified many genes and proteins that play specific roles in the process of systemic metastasis in various types of cancer. Rho GDP dissociation inhibitor 2 (RhoGDI2) has been shown to inhibit metastasis in human bladder cancer, but its role in breast cancer is controversial.
Materials and Methods
We examined the regulation and clinical significance of RhoGDI2 in Korean breast cancer patients by using proteomic approaches.
Results
By using a proteomic approach, we observed an increased expression of RhoGDI2 in human breast cancer tissues when compared to that of the normal breast tissues, and we validated its up-regulation in an independent cohort of 8 breast cancer patients. The clinical implication of a RhoGDI2 expression was investigated in 57 breast cancer patients by performing immunohistochemistry. RhoGDI2 did not show a significant association with the tumor size, lymph node metastasis, the histologic grade or the hormone receptor status. However, the patients with RhoGDI2-expressing tumors had significantly shorter disease-free survival (p=0.043; hazard ratio, 3.87) and distant metastasis-free survival (p=0.039; hazard ratio, 5.15).
Conclusion
Our results demonstrated a potential role of RhoGDI2 as a poor prognostic marker as well as a potential therapeutic target. The pro-metastatic nature of RhoGDI2 shown in our study may indicate its organ-specific role in cancer metastasis.
Introduction
Despite the recent developments in locoregional control and adjuvant systemic treatment for breast cancer patients, up to 40% of these patients ultimately develop metastases in distant organs, and this almost leads to mortalities (1). Tumor metastasis develops in a step-by-step fashion, which involves the serial steps of invasion, arrest in the capillaries, extravasation and formation of clinical metastasis (2). Recent experimental advances have made it possible for researchers to look into the molecular pathways of metastasis and this has brought about the concept of 'metastasis suppressors,' which plays crucial regulatory roles in developing metastases (3-5).
Rho GDP dissociation inhibitors (RhoGDIs) regulate the activity of Rho GTPases, such as Rho, Rac and Cdc42, which play important roles in cell migration and invasion (6). Previous studies using bladder cancer cell lines and tissues have shown that RhoGDI2 inhibits tumor metastasis and it regulates tumor progression in vivo and in vitro (7-9), and this suggested using this molecule as a novel metastasis suppressor (3-5,10). However, recent studies on breast cancer have shown inconsistent results regarding the role of RhoGDI2 for the prognosis and progression of human breast cancer (11,12). Furthermore, it has been shown that RhoGDI2 can promote breast cancer cell invasiveness in vivo (13).
We have recently reported a list of up-regulated proteins by performing a proteomic analysis of human breast cancer tissues, and among the proteins on this list, we observed the differential expression of RhoGDI2 in the cancerous and normal breast tissues (14). In this study, we investigated the expression level and prognostic significance of RhoGDI2 in human breast cancer. Our current study obtained rather contrary results to the aforementioned metastasis suppressing role of RhoGDI2, and this suggests an organ-specific difference in the functions of RhoGDI2.
Materials and Methods
1. Proteomic analysis and western blotting using human breast cancer tissues
Our experimental protocols for proteomic analysis have been previously described (14). Briefly, fresh breast tissue samples were obtained during surgery and these samples were kept in a freezer. The frozen breast tissues were homogenized in homogenization buffer (50 mM Tris-HCl) and a protease inhibitor cocktail (1 mM AEBST, 0.8 mM aprotinin, 21 mM leupeptin, 36 mM bestatin, 15 mM pepstatin A, 14 mM E-64). The total protein concentration was determined by the Bradford method using BSA as a standard.
Isoelectric focusing (IEF) was performed using a PROTEAN IEF cell system (Bio-Rad, Hercules, CA). Protein samples (60 µg protein for the analytical gels or up to 0.5 mg for the micropreparative gels) were dissolved in rehydration solution and this was then applied to IPG strips (18 cm, pH 3-10, Bio-Rad). IEF was initially performed at 250 V for 15 minutes, followed by an increase in voltage to 10,000 V within 3 hours, and this was maintained at 10,000 V until 50 kV were attained. Separation in the second dimension was performed using Protean II xi electrophoresis equipment and Tris-glycine buffer (25 mM Tris, 192 mM glycine; pH 8.3) that contained 0.1% SDS at 5 mA/gel for the initial 1 hour and next 10 mA/gel was used until the bromophenol blue dye marker reached the bottom of the gel. The gels were stained with silver nitrate or Coomassie blue and then the gels were scanned with a high-resolution scanner (GS-710 Calibrated Imaging Densitometer, Bio-Rad, Seoul). The scanned gel image was analyzed using a standard protocol for PDQuest software (Bio-Rad, Seoul). For western blotting, 50 µg of the protein extracts from the breast tissues were run on a 12% SDS polyacrylamide gel, and the separated proteins were electrotransferred onto a polyvinylidene fluoride membrane. The membranes were washed and incubated with anti-RhoGDI-2 rabbit polyclonal antibody (1 : 3,000, Sigma Aldrich, St. Louis, MO) for 1 hour at room temperature. The membranes were then incubated with secondary anti-rabbit IgG (1 : 5,000, Jackson Immuno Research, West Grove, PA) and at last they were developed with ECL (Pierce, Rockford, IL).
2. Immunohistochemistry
From the institution's surgical database, we randomly selected 57 breast cancer patients who underwent an operation at Gyeongsang National University Hospital from January 1999 to November 2002. All the patients underwent a curative operation for breast cancer, and those patients who had surgery for recurrent breast cancer were excluded. The patients for whom the paraffin blocks were not available were also excluded. The formalin-fixed, paraffin-embedded breast cancer tissues of the enrolled patients were collected and the paraffin blocks were sectioned at 4 µm and then mounted on charged slides. The tissues were stained with diluted polyclonal antibody against RhoGDI2 (1 : 2,000, Sigma-Aldrich). Antigen retrieval was facilitated with microwaving the tissue for 30 minutes, and the rest of the staining procedure followed the standard avidin-biotinylated peroxidase complex method. The immunohistochemical stainings were interpreted and categorized using an arbitrary semi-quantitative scale as 0 (negative staining), 1+ (0% to 30% positive) and 2+ (more than 30% positive) by a pathologist. The tumors showing a 1+ or 2+ RhoGDI2 expression were categorized into the positive RhoGDI2 group and the tumors showing a negative RhoGDI2 expression were categorized into the negative RhoGDI2 group for further statistic analysis.
3. Statistical analysis
Statistical analyses were performed by using the two-tailed chisquare test and Student's t-test to the compare nominal and continuous variables, respectively, between the groups. The Cox proportional hazard model was used for survival analysis. All the p-values less than 0.05 were considered statistically significant. The SPSS ver. 11.0 (SPSS Inc., Chicago, IL) was used for all the statistic analyses.
Results
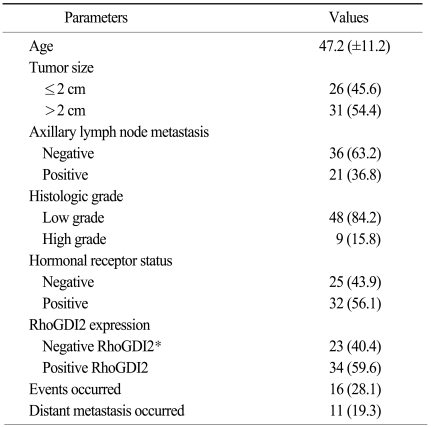
In our previous study, 2-dimentional electrophoresis (2-DE) analysis of the cancer tissues and the normal tissues from 6 breast cancer patients showed a differential expression of RhoGDI2 (14). The RhoGDI2 expression (spot 2218) was up-regulated in all 6 breast cancer tissues when compared to that of their normal counterparts (Fig. 1). This increased expression was statistically significant, as assesed by image analysis of the 2-DE gels (p<0.05). These findings were again validated in an independent cohort comprised of 8 additional operable breast cancer patients by performing western immunoblotting (Fig. 2). While the non-cancerous breast tissues showed variable degrees of a RhoGDI2 expression, the breast cancer tissues showed a distinctly up-regulated expression of RhoGDI2 when compared to that of the non-cancerous tissues (Fig. 2A). Densitometric measurement of the immunoblots showed a statistically significant differential expression between the cancer and normal tissues (p<0.003) (Fig. 2B).
Images of the 2-dimensional electrophoresis (DE) gels of the cancerous and non-cancerous tissues showing increased expression of Rho GDP dissociation inhibitor 2 (RhoGDI2; circle and arrow) (A), and magnified images of the spot representing RhoGDI2 in 6 breast cancer patients (B).

Results of western blotting using and additional 8 invasive ductal carcinoma patients (A) and densitometry results (B).
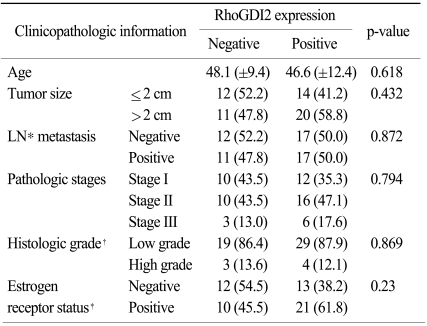
The clinical implication of the RhoGDI2 expression in human breast cancer tissue was investigated by performing immunohistochemical staining for RhoGDI2 in 57 invasive ductal carcinoma patients (Fig. 3). The mean age of the enrolled patients was 47.2 years (±11.2 years). All the patients received curative resection and adjuvant treatments according to the institution's protocols. Among the 57 patients, thirty four patients (59.6%) showed RhoGDI2 positivity on the immunohistochemistry (Table 1). The positive RhoGDI2 group and the negative RhoGDI2 group were compared for various clinicopathologic parameters. The known prognostic factors such as tumor size, axillary lymph node involvement, the histologic grade and the estrogen receptor status did not have any significant association with the RhoGDI2 expression (Table 2).

Immunohistochemical staining for Rho GDP dissociation inhibitor 2 (RhoGDI2) in the human breast cancer tissues RhoGDI2 showed differential expression patterns in the human breast cancer tissues (×400). RhoGDI2 showed both a cytoplasmic expression and a nuclear expression in the human breast cancer tissue; the positive RhoGDI2 expression in 34 patients (A) and the negative RhoGDI2 expression in 23 patients (B).
Comparison of the clinicopathologic characteristics according to the Rho GDP dissociation inhibitor 2 (RhoGDI2) expression status
Univariate survival analysis suggested a potential relationship between the RhoGDI2 expression level and the breast cancer outcomes. Among the 57 patients, 11 patients (19.3%) developed distant metastasis during the follow-up period. Survival analysis using the Kaplan Meier curves showed that the patients with a positive RhoGDI2 expression had significant shorter disease-free survival (p=0.031), and they had a borderline association with distant metastasis-free survival (p=0.09). When adjusted for tumor size, the presence of lymph node metastasis, the histologic grade and the hormonal receptor status, the Cox proportional hazard model showed significant survival differences according to the RhoGDI2 expression. The patients with a negative RhoGDI2 expression had superior disease-free survival and distant metastasis-free survival (Fig. 4, Table 2).

Results of the survival analysis according to the Rho GDP dissociation inhibitor 2 (RhoGDI2) expression The survival curves of the Cox proportional hazard regression analysis show a difference of survival according to the RhoGDI2 expression in terms of disease-free survival (A) and distant metastasis-free survival (B). The adjusted covariates are tumor size, the presence of lymph node metastasis, the histologic grade and the estrogen receptor status. HR, hazard rate; CI, confidence interval.
Discussion
In this study, we show the up-regulated expression of RhoGDI2 in human breast cancer tissues as compared to that of their normal counterparts. Furthermore, by performing immunohistochemical staining for RhoGDI2 in 57 invasive breast cancer tissues, we demonstrated the prognostic significance of RhoGDI2 in breast cancer patients. However, the breast cancer patients with a positive RhoGDI2 expression had shorter disease-free survival and distant metastasis-free survival, and this is a rather contradictory finding to the recently proposed role of RhoGDI2 as a metastasis suppressor molecule (10).
The proposed role of RhoGDI2 as a metastasis suppressor molecule mainly came from the study on bladder cancer by Theodorescu and colleagues (7). They identified RhoGDI2 as an invasion and metastasis suppressor by perfoming a microarray analysis of a bladder cancer cell line model, and they demonstrated the association between a RhoGDI2 expression and the tumor grade or stage in 105 human tumor tissues. Transfection of the RhoGDI2 gene into a highly metastastic bladder cancer cell line resulted in reduced metastatic capability in a mouse model of lung metastasis (7). Subsequently, it was shown that the RhoGDI2 expression is uniformly lower in tumor cell lines when compared with their normal counterparts, and human bladder tumors with a high RhoGDI2 expression had better disease-free survival, and all of this suggested a potent role of RhoGDI2 as a metastasis suppressor (8).
RhoGDI2 can inhibit the dissociation of GDP from various Rho GTPases such as RhoA, Rac1, Rac2 and Cdc42 (6). It has been shown that the expressed levels of RhoA, Rac1 and Cdc42 are higher in breast tumor tissues as compared with that of the normal tissues (15) and these Rho GTPases are involved in breast cancer migration and metastasis both in vitro and in vivo (16,17). Furthermore, it has been observed that RhoGDIs can inhibit cell motility and disrupt the cytoskeleton in other cell types such as fibroblasts and keratinocytes (18,19). Based on this information, the suggested role of RhoGDI2 as a potential metastasis suppressor in breast cancer seemed rationale. Yet the prognostic significance of a RhoGDI2 expression in human breast cancer is currently unknown. Jiang et al. (11) reported that the degrees of RhoGDI2 expression in primary human breast cancer tissues were not associated either disease progression or recurrence.
In contrast to the previous reports (7,8), we observed an increased protein expression of RhoGDI2 in the breast cancer tissues when compared to the normal breast tissues. The pattern of the RhoGDI2 expression in the cancer tissue was consistent among the electrophoresis and western blotting experiments, which included the tissues of 6 and 8 independent patients, respectively, with invasive ductal carcinomas. It was quite interesting that the multivariate survival analysis showed better disease-free survival and distant metastasis-free survival in the patients with tumors showing a negative RhoGDI2 expression, which suggested a pro-metastatic role of RhoGDI2 in breast cancer. Our results are in accordance with the recent study reported by Zhang and Zhang (13) Those authors have shown that the RhoGDI2 expression is up-regulated in highly metastatic breast cancer cell lines such as MDA-MB-231 and a RhoGDI2 expression is absent in a benign mammary epithelial cell line. Furthermore, knockdown of the RhoGDI2 expression by siRNA resulted in decreased cell motility and decreased invasion capability.
Therefore, RhoGDI2 may have different roles for cancer cell progression and metastasis in different tumor types. The tumor type-specific regulatory roles of metastasis suppressors have been shown for other molecules. nm23 is a well-known metastasis suppressor gene for melanoma, breast cancer, colon cancer and oral squamous cell carcinoma (3-5). However, the expression of the nm23 gene has opposite prognostic implications in neuroblastoma, pancreatic carcinoma and acute myeloid leukemia (20-22).
Conclusion
Our study showed an increased expression of RhoGDI2 in human breast cancer tissues, and there was an inverse relationship between the RhoGDI2 expression and tumor recurrence. Our results are contradictory to the previously suggested role of RhoGDI2 as a metastasis suppressor in human cancers. While the prognostic significance of RhoGDI2 in breast cancer needs further validation by a study with a large cohort of patients, our data suggests potential tumor type-specificity for the regulatory function of RhoGDI2 in tumor progression and metastasis.
Notes
This study was supported by the National R&D Program for Cancer Control, Ministry of Health, Welfare and Family Affairs, Republic of Korea (0820050) and the Korea Health Industry Development Institute (A091142).