Clinical Outcome of Gastric Cancer Patients with Bone Marrow Metastases
Article information
Abstract
Purpose
The prognosis of gastric cancer patients with bone marrow metastases is extremely poor. The current study was conducted to evaluate the clinical outcomes of advanced gastric cancer patients with bone marrow metastases.
Materials and Methods
We retrospectively reviewed the medical records of 26 advanced gastric cancer patients with bone marrow metastases who were treated at Soonchunhyang University Hospital between September 1986 and February 2009.
Results
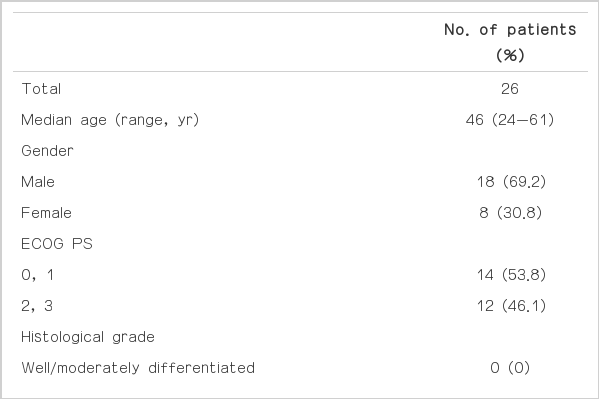
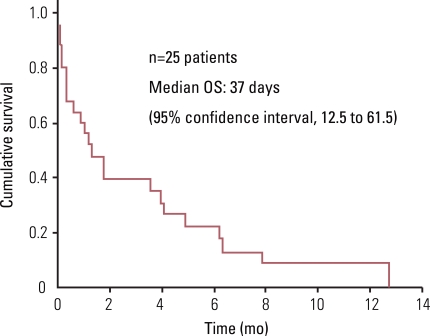
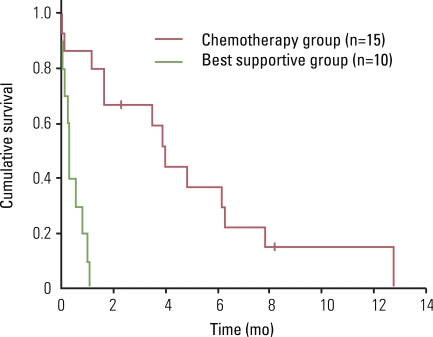
The median age was 46 years (range, 24 to 61 years). All patients had poorly differentiated adenocarcinoma, including 17 signet ring cell carcinomas. The majority of the patients had thrombocytopenia, anemia, and elevated lactate dehydrogenase levels. Sixteen patients (61.5%) received palliative chemotherapy (median, 4 cycles; range, 1 to 13 cycles). The median overall survival after detection of bone marrow metastases for the cohort of patients was 37 days (95% confidence interval, 12.5 to 61.5 days). The median overall survival after detection of bone marrow involvement was 11 days in the best supportive care group (range, 2 to 34 days) and 121 days (range, 3 to 383 days) in the palliative chemotherapy group (p<0.001). The causes of death were tumor progression (11 patients, 45%), brain hemorrhage (6 patients, 25%), infection (5 patients, 21%), and disseminated intravascular coagulation (1 patient, 4%). There were no chemotherapy-related deaths.
Conclusion
Palliative chemotherapy could be considered in advanced gastric cancer patients with bone marrow metastases as a treatment option.
Introduction
Gastric cancer is the most prevalent malignancy in Korea, accounting for 18.3% of all solid tumors [1]. Advanced gastric cancer (AGC) with distant metastases or recurrence remains incurable, with a median survival of 6-9 months. Metastatic gastric cancer remains a therapeutic challenge for medical oncologists, especially for those patients with bone marrow metastases. Disseminated carcinomatosis of the bone marrow by solid tumors frequently originated from gastric origin [2,3], although the actual incidence of this condition is rare. The prognosis of gastric cancer with bone marrow metastases is poor because of a rapidly deteriorating clinical course that is often refractory to conventional treatment [4-6]. Reports on systemic chemotherapy in gastric cancer patients with bone marrow metastases are limited. Indeed, the variety of chemotherapy regimens or supportive care used for these patients reflects the lack of a standard treatment [7,8]. The present retrospective analysis was conducted to identify the clinical features, treatment outcomes, and prognostic factors for survival in gastric cancer patients with bone marrow metastases. We also compared the pre- and post-chemotherapy laboratory changes in gastric cancer patients with bone metastases who received palliative chemotherapy to evaluate clinical improvement, if any.
Materials and Methods
1. Patients and methods
This study retrospectively reviewed the medical records of patients with histopathologically-proven AGC with disseminated carcinomatosis of the bone marrow who were treated at Soonchunhyang University Hospital between September 1986 and February 2009. From the database, 129 AGC patients were identified to have been received a bone marrow biopsy. Of these 129 patients, 26 patients with bone marrow dissemination pathologically-confirmed by bone marrow biopsy were identified.
The following demographic data, disease characteristics, and treatment parameters were reviewed: gender, age, Eastern Cooperative Oncology Group (ECOG) performance status, tumor histology, sites of metastases at the time bone marrow involvement was detected, time elapsed from gastric cancer diagnosis to bone marrow involvement, and laboratory findings (hemoglobin, leukocyte count, platelet count, coagulation profile, and levels of serum lactate dehydrogenase [LDH], serum aspartate aminotransferase [AST], alanine aminotransferase [ALT], serum alkaline phosphatase [ALP], albumin, sodium, calcium, total bilirubin, and uric acid). The study was performed after approval by the Institutional Review Board of the Soochunhyang University Hospital in Seoul, Korea.
2. Statistics
The primary endpoint of the current study was overall survival (OS), calculated using the Kaplan-Meier product-limit method. OS was defined as the time elapsed from the date bone marrow involvement was detected to the date of death. Comparison of survival by univariate analysis was estimated by the log-rank test and Cox's proportional hazard model was used for multivariate analysis. A p-value<0.05 was considered to be statistically significant, but clinical parameters with a p-value<0.10 were included in the multivariate analysis. Laboratory variables were initially recorded as continuous variables, and subsequently dichotomized according to the median value of each variable.
Results
1. Patient characteristics
The clinical characteristics of all patients are listed in Table 1. The median age was 46 years (range, 24 to 61 years) and the male-to-female ratio was 2 : 1. The ECOG performance status was >2 in 12 patients (46.2%). All of the patients had poorly differentiated adenocarcinoma (34.6%) or signet ring cell adenocarcinoma (65.4%). The most common site of metastasis was bone (57.7%), followed by lymph nodes (46.2%), lungs (11.5%), liver (3.8%), and peritoneum (3.8%). The most common abnormal laboratory findings were anemia and thrombocytopenia. Reasons for bone marrow biopsy were thrombocytopenia, anemia, and leukoerythroblastic reactions. An elevation in the serum ALP and LDH levels was a characteristic clinical finding. The median calcium level was within the normal range. Of 26 patients, 16 (61.5%) received palliative chemotherapy, as follows: platinum-based (n=4), taxane-based (n=1), irinotecanbased (n=3), and 5-fluorouracil-based (n=8).
2. Response and survival
Sixteen patients (61.5%) received palliative chemotherapy, with a median of 4 cycles per patient (range, 1 to 13 cycles). One patient discontinued treatment and was lost to follow-up, so 15 patients were analyzed in this study. The objective response rate was 33.3%; 5 patients (33.3%) had partial responses, 5 patients (33.3%) had stable disease, and 3 patients (20.0%) had progressive disease. The disease control rate was 66.6% (Table 2). The median survival time in patients with gastric cancer and bone marrow metastases was 37 days (95% confidence interval [CI], 12.5 to 61.5 days). The median survival time was 11 days in the supportive care group (range, 2 to 34 days) and 121 days (range, 3 to 383 days) in the palliative chemotherapy group (p<0.001) (Figs. 1 and 2). Patients died of tumor progression (11 patients, 45%), brain hemorrhage (6 patients, 25%), infection (5 patients, 21%), and disseminated intravascular coagulation (1 patient, 4%). There were no chemotherapy-related deaths.
3. Laboratory changes before and after chemotherapy
White blood cell (WBC) and platelet counts, hemoglobin, prothrombin time (PT), and activated partial thromboplastin time (aPTT) were checked at every cycle of chemotherapy; the median post-chemotherapy laboratory values were obtained after 3 cycles of chemotherapy. The WBC and platelet counts were increased in the chemotherapy group, but hemoglobin, PT, and aPTT values did not change significantly (Table 3).
4. Prognostic factor analysis
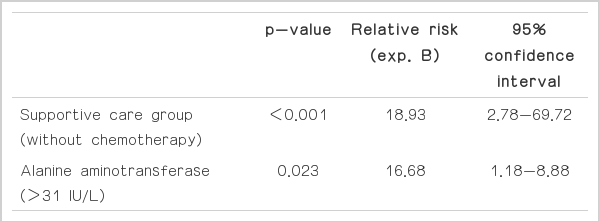
Based on univariate analyses, WBC>8,000/µL (p<0.02) and supportive care (p<0.001) had adverse effects on survival (Table 4). Clinical parameters with a p-value<0.10 (WBC>8,000/µL, ALT>31 IU/L, and supportive care) and universal parameters, such as ECOG and age, were included in the multivariate analysis. Using the forward Cox regression model, statistically significant poor prognostic factors for survival were supportive care (p<0.001; relative risk [RR], 18.93; 95% confidence interval [CI], 2.78 to 69.72) and an ALT>31 IU/L (p<0.023; RR, 16.68; 95% CI, 1.18 to 8.88) (Table 5).
Discussion
When compared with supportive care, chemotherapy for advanced or recurrent gastric cancer has survival benefits [9,10]. The incidence of gastric cancer patients with bone marrow metastases is low. As a result, the clinical features and optimal treatment options for such patients have yet to be established. Using retrospective single center data, Kim et al. [11] reported the prevalence of AGC with bone marrow metastases, as confirmed by bone marrow biopsy, to be 0.024%. In that study, the significant poor prognostic factors for survival were serum Na<133 mmol/L, lung metastases, and peritoneal seeding. Because this subset of patients was excluded from clinical trials due to inadequate laboratory findings or other causes, little outcome data are available on the clinical features of this group of patients or the optimal chemotherapy regimens.
The aim of the current study was to analyze the clinical features, treatment outcomes, and prognostic factors for survival in gastric cancer patients with bone marrow metastases. The clinical features of such patients include a younger age, an elevation in the level of serum ALP and/or LDH, extensive bone metastases, low incidence of hypercalcemia, and histologic type gastric cancer (signet ring cell carcinoma or poorly differentiated adenocarcinoma) [7,11]. In the present study, the majority of the patients were characterized by young age (median age, 46 years), poorly differentiated adenocarcinoma (100%), elevated LDH (1,330 IU/L; normal range, 279 to 2,590 IU/L), and elevated ALP (467 IU/L; range, 30 to 2,352 IU/L). The clinical factors predicting survival based on multivariate analysis were chemotherapy and elevated ALT levels. However, the numbers of analyzed cases were too small to derive any definite conclusion about the association of chemotherapy with survival in patients with bone marrow metastases.
In the present study, the median survival for the entire cohort of patients was 37 days (95% CI, 12.5 to 61.5 days), which is consistent with the findings from previous studies [7,11]. Thus, the prognosis of AGC patients with bone marrow metastases is poor, regardless of the treatment, considering that the median survival for patients with metastatic or inoperable AGC is 6-9 months.
The median survival time was 11 days in the supportive care group (range, 2 to 34 days) and 121 days (range, 3 to 383 days) in the palliative chemotherapy group (p<0.001). Kusumoto et al. [7] studied nine patients with gastric cancer and diffuse bone marrow metastases; the median survival time was 187±62 days in the chemotherapy group (n=4) and 55±42 days in the supportive group (p<0.02). Similarly, Kim et al. [11] studied 39 patients with gastric cancer and bone marrow metastases; the median survival from the time of detection of bone marrow involvement was 44 days (range, 2 to 252 days) in all patients, 20 days (range, 2 to 137 days) in the best supportive group, and 67 days (range, 5 to 252 days) in the palliative chemotherapy group (p=0.026). Although limited by a relatively small number of patients and the retrospective design of the study, the survival period of the patients treated with chemotherapy was significantly longer than the patients who did not receive chemotherapy. The results suggest that palliative chemotherapy should be considered as a method of treatment to improve the prognosis of gastric cancer patients with bone marrow metastases. However, due to the lack of prospective studies for gastric cancer patients with bone marrow metastases, an optimal regimen of chemotherapy is not known.
In the present study, the laboratory findings at the time of detection of bone metastases were as follows: median WBC, 8,200/µL (range, 2,000 to 5,350/µL); median hemoglobin, 8.9 g/dL (range, 5.0 to 13.6 g/dL); and platelet count, 56,000/µL (range, 16,000 to 411,000/µL). Thirty percent of patients had inadequate laboratory findings for cytotoxic chemotherapies. Nevertheless, the most common cause of death in patients with gastric cancer and bone marrow metastases was disease progression, not chemotherapy-related hematologic toxicities. But, it is difficult to determine whether the intensity of cytotoxic chemotherapy should be increased to prolong patient survival or lowered to avoid toxicity. Thrombocytopenia (80.8%) was the most common peripheral blood finding. At the time of diagnosis with AGC, clinicians should suspect bone marrow metastases in gastric cancer patients and consider a bone marrow biopsy if patients present with prominent thrombocytopenia. In comparing the laboratory changes before and after chemotherapy, an increased WBC count and hemoglobin level were statistically insignificant, but thrombocytopenia improved significantly with better outcomes, such as reducing the need for platelet transfusion.
The optimal treatment strategy for AGC patients with bone marrow metastases remains to be determined due to the lack of prospective studies. Based on our analysis, chemotherapy in gastric cancer patients with bone marrow metastases seems to be efficacious in terms of OS. Given these considerations, our next step will be to a prospective investigation of chemotherapy in the treatment of AGC patients with bone marrow metastases.
Conclusion
This study suggests that palliative chemotherapy could be considered as a treatment option in AGC patients with bone marrow metastases. The optimal treatment modality needs to be defined in prospective trials for this subset of patients.
Notes
Conflict of interest relevant to this article was not reported.