Primary Follicular Lymphoma in a Male Breast: A Case Report
Article information
Abstract
Primary breast lymphoma (PBL) is a rare disease, particularly in males. Diffuse large B cell lymphoma is the most common PBL, while follicular lymphoma is less common. Furthermore, primary follicular lymphoma of a male breast is rarely reported. We report a male patient with primary follicular lymphoma of the breast and hepatocellular carcinoma (HCC). A 46-year-old man was diagnosed with liver cirrhosis secondary to chronic hepatitis B infection. Ten years later, he underwent segmentectomy of the liver due to HCC. Another 5 months later, he presented with a painless mass in the right chest wall. The mass was diagnosed as follicular lymphoma of the breast. The stage was IEA and he did not receive adjuvant therapy. Although only a few cases have been reported, lymphoma should be considered as a possible cause of breast mass, even in male patients.
Introduction
Breast lymphoma is a rare disease due to the small amount of lymphoid tissue in the breast. According to the definition of Wiseman and Liao [1], primary breast lymphoma (PBL) is diagnosed when the first major manifestation site of lymphoma is the breast without involvement of any other organ. Due to this relatively narrow definition, PBL represents less than 1% of all non-Hodgkin's lymphomas (NHL) and 1.7% to 3% of all extranodal NHLs [2]. The most common histologic type of PBL is diffuse large B cell lymphoma (DLBL), while follicular lymphoma (FL) is less common. Therefore, the incidence of primary breast FL in males is extremely rare. Here, we report a male patient who had hepatocellular carcinoma (HCC) due to cirrhosis and developed primary breast FL.
Case Report
A 46-year-old man was diagnosed with liver cirrhosis secondary to chronic hepatitis B infection. Ten years later, a mass with 1.5 cm diameter was detected by abdominal computed tomography (CT) scan in Couinaud's segment 8 during a routine follow-up. Viral serologic tests were positive for serum hepatitis B surface antigen (HBsAg), envelope antigen (HBeAg), and antibody (HBeAb) and negative for hepatitis C virus antibody. The patient underwent segmentectomy of the liver. Histology of the resected specimen revealed 1.2 cm HCC with Edmondson grade II and peritumoral microvessel invasion (pT2). The postoperative course was uneventful.
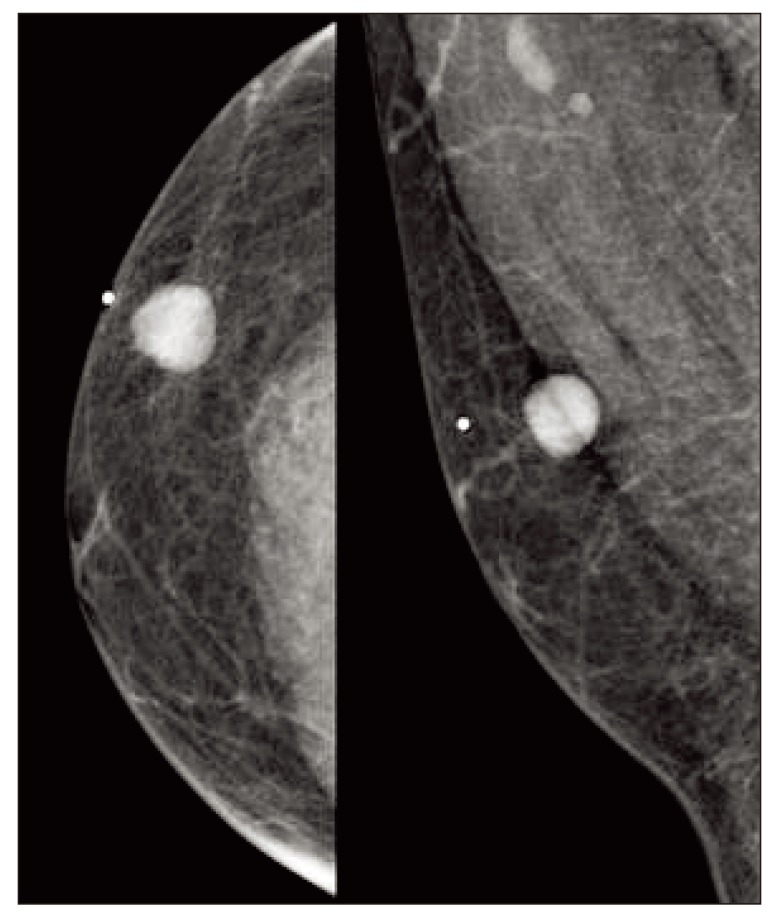
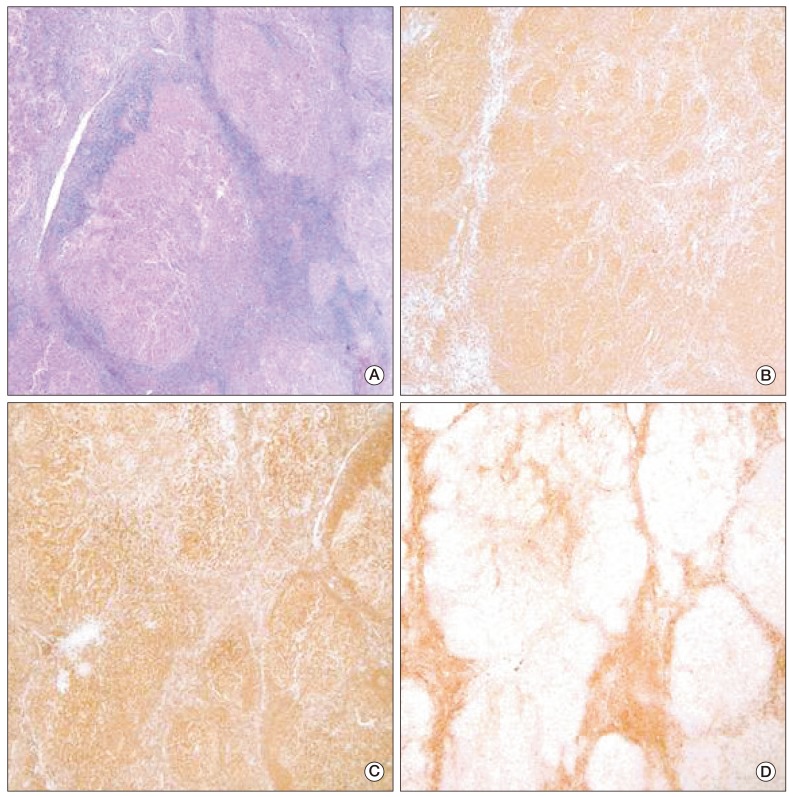
Five months later, the patient presented with a painless palpable mass in the right chest wall. The mass was movable and round shaped without fixation to the skin or muscle. On mammography, there was a round, homogenous, well-circumscribed mass (Fig. 1). Ultrasonography revealed an oval hypoechoic mass, measuring 1.6 cm in diameter, on the right chest wall without evidence of axillary involvement. Excisional biopsy was performed for histologic examination. Microscopic findings showed atypical lymphoid cells with a follicular growth pattern. Immunohistochemical staining was positive for antibodies against CD20, B cell lymphoma (Bcl)-2, and Bcl-6, and negative for CD3 (Fig. 2). Definite histology was diagnosed as grade 1 FL of the breast based on morphologic features and immunohistochemistry. Positron emission tomography-CT, abdominal CT, and chest CT were used to stage the disease, and there were no other suspected lesions. Aspirate and bone marrow biopsy was performed for initial staging procedure, and the results revealed normocellular marrow with no evidence of malignancy. The stage was IEA according to the Ann Arbor staging system [3], and the prognostic index was low according to the Follicular Lymphoma International Prognostic Index (FL-IPI) [4]. The patient did not receive further adjuvant therapy, and he remains alive with complete remission at 40 months after surgery.
Discussion
About one-fourth of NHL patients present with an extranodal origin, which can arise from almost any organ in the body [5]. However, because of the paucity of lymphoid tissue in the breast, primary and secondary involvements of the breast by lymphoma are rare. DLBL is a most common histotype of PBL, while indolent lymphomas, particularly FL, are common in secondary breast lymphomas [6]. PBLs are rare in males because of the extensive involution of the male breast gland. Here we report an interesting case that suggests that the male breast can be the primary site of extranodal NHL. To the best of our knowledge, this is the first case report of a primary FL in a male breast. There have only been 2 cases of FL in the male breast. One was a case of secondary breast involvement by systemic disease [7] and the other case was described as a patient in a study cohort without detailed information [8].
Viral hepatitis and alcoholic liver disease are major risk factors for liver cirrhosis and HCC. According to recent studies, patients with liver cirrhosis are not only at an increased risk of HCC, but also at an increased risk of extrahepatic cancers [9]. Breast, lung, and colon cancers and lymphoma are common extra-hepatic cancers. Although the mechanisms by which hepatitis B virus (HBV) might induce NHL are unclear, recent evidence suggests that chronic B-cell activation promotes DNA damage and transformation into NHL [10]. The relationship between HBV infection and various NHL subtypes has not established. In a large cohort study of 53,045 patients with HBV infection [11], HBsAg-positive patients had an increased risk of DLBL, while associations with follicular and T-cell NHL were not significant. In this case, although the patient developed HCC due to liver cirrhosis, the relationship between FL and liver cirrhosis is unclear.
Due to its rarity, the prognosis and standard treatment of primary FL of the breast remains unclear. Compared with other NHLs, indolent FL is characterized by prolonged survival [12]. In a report of 36 patients with primary breast FL [8], the median overall survival period was 8.1 years, and the median progression-free period was 3.9 years. In general, primary breast FL follows treatment recommendations for FLs in other location. Surgery is only recommended for diagnostic purposes, and extensive surgery may delay beginning of adjuvant treatment [8]. Combination chemotherapy and combined modality treatment are matters of debate. Some reports suggest that immediate treatment is better for prognosis [13], however, a number of reports suggest that a "Watch and Wait" strategy is feasible in selected patients [14]. In our case, the tumor was stage IEA and the FL-IPI was low. Further, he had liver cirrhosis and had undergone segmentectomy of liver due to HCC. We chose a "Watch and Wait" strategy, and the patient remains alive and in complete remission.
In conclusion, we describe the first reported male patient with primary breast FL. Although only a few cases have been reported, lymphoma should be considered as a cause of breast mass especially in a cirrhotic liver patient. Treatment modalities for FL are controversial, but the "Watch and Wait" strategy was effective for our patient.
Notes
Conflict of interest relevant to this article was not reported.