Introduction
Sarcoma is a heterogeneous group of cancers of the soft tissue and bone, and some are extremely rare. The rarity and diverse characteristics of sarcomas has impeded the development of new treatments. Doxorubicin and ifosfamide, either alone or in combination, have been the backbone of metastatic soft tissue sarcomas (STS) therapy for over 15 years. Few effective chemotherapeutic options for the treatment of metastatic STS beyond 1st line treatment with doxorubicin- and ifosfamide-based regimens exist. Because alternative regimens for the effective treatment of metastatic sarcoma are unavailable, varying combinations, including doxorubicin and/or ifosfamide, are sometimes used to treat patients who either not candidates for use or are resistant to treatment with doxorubicin and/or ifosfamide. Thus, a number of clinical trials have been conducted to explore alternative chemotherapeutic options.
The reported response rate (RR) from five phase II trials of single-agent gemcitabine treatment in patients with soft tissue and bone sarcoma was 3-18% (median RR, 5.5%) [1,2]. Docetaxel as a single agent (100 mg/m2, IV every 3 weeks) was examined as a second line therapy in a phase II study conducted by the European Organization for Research and Treatment of Cancer (EORTC) [3]. However, based on the results of several phase II trials, the efficacy of single-agent docetaxel treatment in treating STS appears to be quite limited with a reported RR of 0-18% (median RR, 15%) [3-6].
Although use of gemcitabine or docetaxel as a single agent has limited efficacy, their use in combination has been shown to be effective in patients with STS. A clinical study by Hensley et al. [7] reported an impressive RR of 53% in patients with predominantly uterine leiomyosarcoma using a study regimen consisting of gemcitabine at a dose of 900 mg/m2 given over a 90-minute IV infusion on days 1 and 8, and docetaxel at a dose of 100 mg/m2 given over a 60-minute IV infusion on day 8, followed by granulocyte-colony stimulation factor (G-CSF), during a 21 day period [7]. In a subsequent retrospective study of 35 patients with a variety of histologic subtypes of adult sarcoma, the patients received a similar regimen but with lower dosages of gemcitabine (675 mg/m2). These patients had previously received doxorubicin and/or ifosfamide, or were patients for whom use of gemcitabine and docetaxel (GD) was preferable for other medical reasons. The results of the trial demonstrated an overall RR of 43% and the responses included patients with osteosarcoma and Ewing's sarcoma [8]. The combination of GD also has been evaluated for use in treatment of a variety of malignancies, with activity demonstrated in non-small cell lung cancer (NSCLC), breast cancer, esophageal cancer and others [9,10].
Recently, a multi-institutional, randomized phase II study in adult metastatic STS patients demonstrated superior progression-free survival (PFS) and overall survival (OS) for use of the combination regimen when compared with use of gemcitabine alone. However, more than 40% of patients receiving a combination of GD needed to discontinue the treatment within 6 months of therapy due to a variety of toxicities, despite adhering to recommended dose reductions [11].
Therefore, to identify the optimum dose and schedule that has both an antitumor effect and acceptable toxicity, we administered weekly gemcitabine-docetaxel treatments to patients with metastatic or recurrent STS who had failed to improve using doxorubicin and ifosfamide combination chemotherapy. Here, we report the results of our retrospective analysis of 22 patients with advanced and refractory STS.
Materials and Methods
From September 2008 to March 2011, a total of 22 patients with a variety of STS were treated at Yonsei Cancer Center (Table 1), and the medical records of these patients were retrospectively reviewed. All patients had metastatic or recurrent cancer and had previously received doxorubicin and ifosfamide combination chemotherapy.
Following a 21 day cycle, on days 1 and 8, patients received 1,000 mg/m2 gemcitabine intravenously over a 100 minutes duration, followed immediately by 35 mg/m2 intravenous docetaxel over 60 minutes. Standard prophylactic premedications were provided on day 1 and day 8 of diphenhydramine and ondansetron or its equivalent. In addition, patients received 8-15 mg intravenous dexamethasone, 30 minutes before docetaxel was administered.
In almost all patients, radiological evaluation consisted of a computed tomography scan and bone scintigraphy, as indicated. Response was assessed every two to three cycles. When measurements were available, radiographic studies and/or reports were reassessed and responses were classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) [12]. Toxicities were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0.
RR was reported on an intent-to-treat basis. Best overall response was defined as the best response designation recorded from the start of treatment until disease progression. PFS was defined as the time from first treatment until clinical or radiographic progression, or death. OS was reported as the date of first treatment until death by any cause. PFS and OS were calculated using Kaplan-Meier curves. Differences in survival between subgroups were compared using the log-rank test.
Results
1. Patient characteristics
Patients in the study included 12 females and 10 males with a median age of 41.5 years (range, 22 to 67 years). The patient characteristics are shown in Table 1.
All patients had previously received doxorubicin and ifosfamide combination chemotherapy with a palliative aim. Of the 22 patients, 20 were considered to be resistant to both drugs and 2 were unable to tolerate these drugs due to hematologic toxicity. Weekly gemcitabine-docetaxel was delivered as a second line chemotherapy to 14 patients (63.6%) and as a third line or more for 8 patients (36.4%).
2. Treatment delivery
A median of 3 weekly gemcitabine-docetaxel chemotherapy courses were delivered (range, 1 to 39 cycles) with a total of 116 courses. No major side effects or severe complications that could be attributed to treatment were reported. According to the medical records, only 4 patients (18.2%) needed a dose reduction due to hematological toxicity. The primary justification for therapy discontinuation was progressive disease (PD) in 20 patients. Of the other 2 patients, one withdrew due to grade 2 gastroparesis and one suffered acute renal failure due to tumor-associated obstructive uropathy. The relative dose intensities per cycle were 90.5% for both gemcitabine and docetaxel. Prophylactic G-CSF was not used for any patients, while therapeutic G-CSF was used for patients with febrile or grade 4 neutropenia.
3. Toxicity
Treatment among the patients was generally well tolerated. Among those exhibiting hematologic toxicity, grade 3-4 neutropenia occurred in 18.2% of cases and grade 3-4 thrombocytopenia occurred in 13.6% of cases. Grade 3-4 mucositis occurred in 4.5% of cases. No patient discontinued treatment due to toxicity or died of treatment-related toxicity. Only 4 patients (18.2%) required dose reduction, primarily due to myelosuppression (Table 2). An allergic reaction occurred with 6 patients when docetaxel was administered, and in these cases, the infusion of docetaxel was discontinued until the symptoms improved and then infusion was slowly restarted. All patients were able to continue their therapy.
4. Efficacy
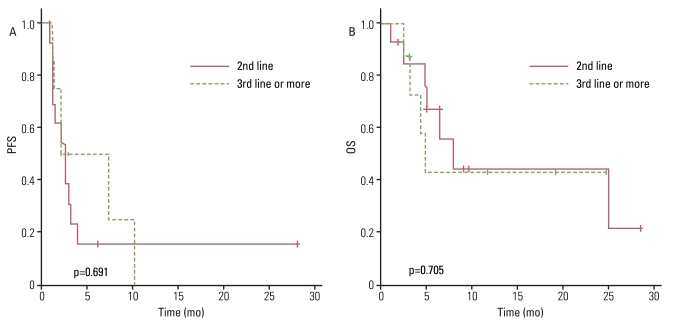
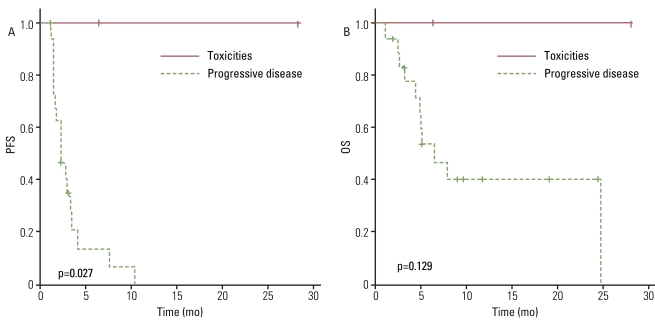
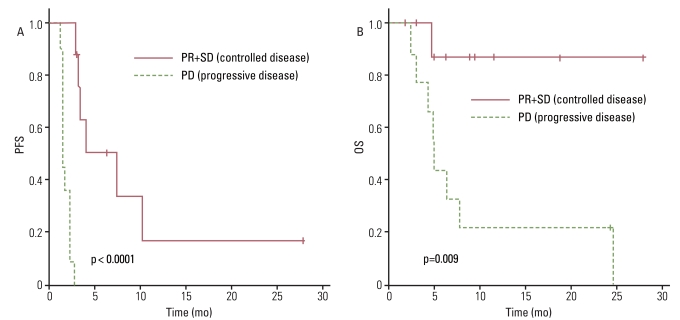
The antitumor effect was reported on an intent-to-treat basis. All patients had at least one measurable lesion. Of the 22 patients reviewed, 2 were not evaluated due to untimely death and one patient was transferred to another hospital prior to evaluation. Among the 22 treated patients, one patient (4.5%) with uterine leiomyosarcoma had a partial response (PR), 8 patients (36.4%) had the best response of stable disease (SD), and 11 (50%) had PD. Thus, an overall RR of 4.5% was observed while the disease control rate (DCR) was 40.9% with a median follow-up of 6.1 months (DCR: leiomyosarcoma, 66.7%; non-leiomyosarcoma, 31.3%). The median PFS was 2.7 months (95% confidence interval [CI], 2.0 to 3.4 months) and the 12-week PFS rate was 41.9% (Fig. 1A). We performed univariate analyses and did not observe any statistical association between PFS and clinical parameters including age, sex, initial localization, prior treatment at baseline, Eastern Cooperative Oncology Group performance status (ECOG PS), histology, or previous lines of chemotherapy before the GD combination (second line vs. third line or more) (Fig. 2A). However, PFS was correlated with the justification for the prior chemotherapy regimen change (toxicities vs. PD; p=0.027) (Fig. 3A) and the treatment response to weekly GD treatment (PR and SD vs. PD; p<0.0001) (Fig. 4A). Median PFS was 4.0 months (range, 2.5 to 28.2 months) in patients achieving disease control (PR and SD), compared with 1.4 months (range, 1.2 to 2.7 months) in those not achieving disease control.
The median survival duration was estimated to be 7.8 months (95% CI, 0.0 to 20.6 months). The six-month survival rate was 58.7% and the one-year survival rate was estimated to be 45.7% (Fig. 1B). No statistical association was observed between OS and clinical parameters including age, sex, initial localization, prior treatment at baseline, ECOG PS and justification for the prior chemotherapy regimen change (Fig. 3B).
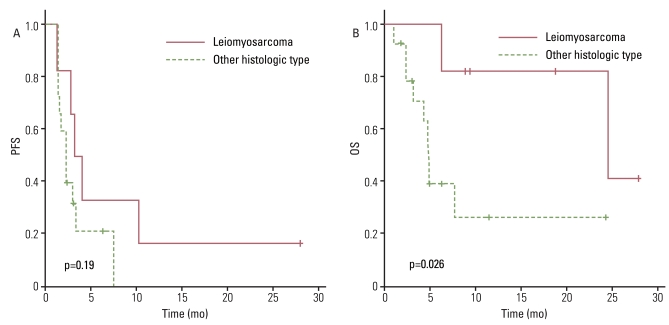
Interestingly, there was no significant difference in OS between patients receiving weekly GD as a second line of treatment and those who received a third line or more of chemotherapy (Fig. 2B). However, OS did correlate with histology, suggesting patients with leiomyosarcoma have a higher survival rate than other histological subtypes (p=0.026) (Fig. 5A and B). Median OS was 24.7 months (range, 6.4 to 28.2 months) in patients with leiomyosarcoma, compared with 4.9 months (range, 1.1 to 24.4 months) in those with other histology subtypes. Treatment response to weekly GD treatment (PR and SD vs. PD) (p=0.009) also correlated with OS (Fig. 4B), and disease control (PR and SD) may be predictive of survival.
Discussion
Currently, the combination of doxorubicin and ifosfamide is a standard chemotherapeutic regimen for soft tissue sarcoma patients with a good performance status. However, some patients are not candidates for this therapy for reasons such as underlying cardiac dysfunction, renal insufficiency or poor performance status. Furthermore, effective chemotherapeutic options for the treatment of metastatic sarcoma, beyond 1st line treatment with doxorubicin- and ifosfamide-based regimens, remain limited.
Gemcitabine (20,20-diflurodeoxycitidine) is a fluorinated analog of the nucleoside, deoxycytidine. The active form, obtained by phosphorylation, inhibits ribonucleotide reductase as a diphosphate, while the triphosphate is incorporated into DNA and blocks DNA synthesis [13-16]. Docetaxel stabilizes tubulin and inhibits mitotic and interphase cellular functions [17,18]. Therefore, combination of GD may provide synergistic effects due to their complementary mechanisms of action whereby gemcitabine induces cell cycle arrest and docetaxel promotes cell death [19]. The combination of GD has been shown in recent studies to be effective in patients with STS [7,8,11,20]. In these studies, IV infusion of docetaxel at a dose of 100 mg/m2 was administered every 3 weeks. Generally, the docetaxel dose was used to treat patients with NSCLC, breast cancer and others as a one hour IV infusion administered once every 3 weeks (3-weekly) at a dose ranging from 60-100 mg/m2. In Asia, conventional 3-weekly doses of 60-75 mg/m2 have typically been administered due to decreased tolerability compared to non-Asian patients [21]. However, in the 3-weekly docetaxel schedule, neutropenia occurs in virtually all patients, and grade 3/4 neutropenia occurs in 65-75% of patients given 60-75 mg/m2. Hence, several clinical trials have examined docetaxel administered as weekly doses of 35-40 mg/m2, which demonstrated significantly lower toxicity profiles, especially for hematologic toxicity, as well as efficacy similar to the 3-weekly schedule [22-25]. Our patients were heavily treated, and we administered docetaxel at a weekly dose of 35 mg/m2.
In a phase II trial including 29 patients with unresectable uterine leiomyosarcoma and 5 patients with STS of other primary sites, an overall RR of 53% (95% CI, 35 to 70%) with acceptable toxicity results was very encouraging. This study was impressive in its demonstration of leiomyosarcoma as a subtype that is relatively sensitive to this chemotherapy combination [7]. Several subsequent studies have observed antitumor activity using combinations of GD in patients with leiomyosarcoma. Therefore, the efficacy of GD depends on histology. While the OS was superior in patients with leiomyosarcoma compared to those with other histological subtypes in our study (p=0.026) (Fig. 4B), it is not known why leiomyosarcoma and malignant fibrous histiocytoma/high-grade undifferentiated pleomorphic sarcoma respond better to this combination [8,11,20].
We anticipated the response or survival of patients who received weekly GD as a second line to be superior than those who received it as third line or more. Interestingly, there was no significant difference in the OS between the two groups (Fig. 2B). We suspect many patients who received GD as a third line or more had leiomyosarcoma. But, almost all patient who received it as a third line or more had non-leiomyosarcoma (87.5%), and only one patient had leiomyoma (12.5%). In other studies, the overall RR or OS was similar both for patients who had received prior treatments and for those who were treated with GD as a first line therapy [7,8,20]. The reason of this point is not clear, but we suppose it's because the combination of GD have antitumor effect in a different way than other chemotherapeutic agents (doxorubicin and ifosfamide), and the patents with relatively good performance received third line treatment.
Our weekly GD treatment was well tolerated, and grade 3-4 neutropenia and thrombocytopenia occurred in only 18.2% and 13.6% of cases. In previous trials, the rate of grade 3-4 neutropenia and thrombocytopenia were 20-40%, though those who were treated with GD as a 1st line therapy were included [7,11]. In comparison, our study demonstrated an extremely low toxicity profile. Also, no major side effects or severe complications that could be attributed to weekly GD administration were reported. This suggests that a weekly divided dose of docetaxel may be more tolerable. In addition, there may be a possibility of increasing dose intensity with proper G-CSF support.
Conclusion
Weekly infusions of GD were very well tolerated and showed moderate efficacy as second and third line treatments, indicating that this could be a reasonable salvage treatment option for metastatic STS, refractory to doxorubicin and ifosfamide. The fact that the weekly GD treatment in this study resulted in better survival for patients with leiomyosarcoma highlights the importance of selecting histology subtype-specific treatment. The fact that there was no significant difference in OS between the patients receiving second or third line treatment or more, illustrated the possibility for use of weekly GD as an effective salvage treatment for heavily treated metastatic STS patients. However, fewer patents were enrolled in this study than in the other studies, and our patients were selected based on good performance and organ function for this salvage treatment, criteria which could become the basis for a prospective validation study. With the result of our study in mind, future salvage treatment studies should be conducted for third line or greater patients, including a higher prophylactic G-CSF treatment dose in patients with proper host status.