AbstractLocally advanced non-small cell lung cancer (NSCLC) is a heterogeneous disease, and we have embarked on an era where patients will benefit from individualized therapeutic strategies based on identifiable molecular characteristics of the tumor. The landmark studies demonstrating the importance of molecular characterization of tumors for NSCLC patients, the promising molecular pathways, and the potential molecular targets/agents for treatment of this disease will be reviewed. Understanding these issues will aid in the development of rationally designed clinical trials, so as to determine best means of appropriately incorporating these molecular strategies, to the current standard of radiation and chemotherapy regimens, for the treatment of locally advanced NSCLC.
IntroductionSignificant advances have been made in the treatment of locally advanced non-small cell lung cancer (NSCLC) over the past few decades. Many of the advances in the treatment of this disease have come from the works involving combined modality therapy, where the combination of chemotherapy with radiation treatments have made a significant impact in the outcome of these patients [1]. However, despite encouraging improvements in survival over the past few decades, the absolute overall survival (OS) and the outcome of the patients with locally advanced NSCLC remain poor. Active areas of research aimed at further improving the outcomes include determining appropriate sequencing of systemic treatments with radiation therapy (RT) in the combined modality setting, discovery of novel agents, and technological advances aimed at improving delivery of radiation treatments. One of the most intriguing and exciting discoveries includes the identification of molecular pathways and designing of targeted agents based on such pathways. For advanced NSCLC, we have now come upon an era where patients will benefit from the individualized therapeutic strategies based on the identifiable molecular characteristics of tumor, which should lead to better outcome and more effective clinical trial design. In depth classification of a patient's tumor molecular profile should, among other things, aid in the selection of the appropriate targeted agents to be used for that specific patient. This notion is becoming more and more attractive based on some of the landmark studies demonstrating the importance of molecular characterization of tumors in NSCLC patients, as reviewed below. Such works have ushered in a new era of clinical trial design, where molecular therapeutics, molecular diagnostics/profiling, and biomarker-based patient selection strategies have become integral components of current clinical trial development. Sobering, however, is the statistics reported from review of clinical trials for NSCLC worldwide in 2009 [2]. This study demonstrated that, although biomarker analysis was included in 37.5% of clinical trials for NSCLC, only 7.9% of these trials used biomarkers to actively select patients for the trials [2]. As we stand at the gateway of personalized medicine era for treatment of NSCLC patients, there is no doubt that we need to improve upon these statistics. In this article, we will review some of the different molecular therapeutic strategies that have made significant impact for NSCLC patients, and how these strategies may be integrated into combined modality therapy of locally advanced NSCLC patients by development of rationally designed clinical studies.
Molecular Therapeutic Agents for NSCLCRecent discoveries in molecular biology have identified a number of molecular pathways that may be responsible for the etiology of cancer cell development, cancer cell progression and growth, and resistance of cancer cells to radiation or other cytotoxic agents. Therefore, these pathways are being explored as potential targets for augmentation of radiation response or chemotherapy response. Some of these agents have also been tested for use as primary therapy for lung cancer patients meeting the appropriate molecular profile. Truly in the last decade, there has been an explosion of new molecular targeted agents for use in lung cancer therapy. The challenge for a radiation oncologist in this molecular era is determining what would be the best method of integrating cytotoxic radiation treatments to a molecular based therapeutic plan.
Among this expanding list of molecular targets for NSCLC includes epidermal growth factor (EGF) and its receptor (EGFR), vascular endothelial growth factor (VEGF) and its receptor (VEGFR), anaplastic lymphoma kinase (ALK) fusion protein (EML4-ALK), B-Raf, PIK3CA gene, ErbB2(Her2/neu) amplification or mutant genes, mammalian target of rapamycin and various other molecules that regulate different steps in their signal transduction pathways [3]. While the preclinical data would suggest that all of these are extremely important and viable targets to be exploited in improving therapeutic efficacy, not all agents have proven to be beneficial in the clinical setting. A handful of agents have now gained United States Food and Drug Administration (FDA) approval for cancer therapy, while many other agents are undergoing clinical trials to determine their efficacy when used in combination therapy with other cytotoxic agents, including ionizing radiation. Some agents are potentially single pathway targeting agents, and others are able to target multiple molecular signaling pathways. The most clinically advanced of these strategies include agents targeting EGFR, VEGF/VEGFR, and ALK1 pathways. Due to the broad scope of this topic, we will primarily focus on these molecular agents that have received FDA approval. Molecular agents that have been studied in conjunction with radiation treatments in the clinical setting are outlined in Table 1.
1. EGFRTargeting EGFR is one of the model paradigms for development of combining RT with molecular based therapeutics. EGFR is also known as ErbB1, a member of the ErbB family of receptor tyrosine kinases which also includes ErbB2 (HER2/neu). EGFR is a 170-kD transmembrane glycoprotein with an intracellular domain possessing intrinsic tyrosine kinase activity. On binding to a ligand, such as EGF or transforming growth factor-α, EGFR undergoes autophosphorylation and initiates transduction signals regulating cell division, metastases, angiogenesis, proliferation, and differentiation. EGFR plays an important role in tumor growth and response to cytotoxic agents, including ionizing radiation. The receptor is frequently expressed in high levels in many types of cancer, which is often associated with more aggressive tumors, poor patient prognosis, and tumor resistance to treatment with cytotoxic agents including radiation [4-8]. In vitro experimental studies have provided solid evidence linking EGFR with resistance to cytotoxic drugs [5,9-11]. In vivo studies have shown that blockade of EGFR, such as with cetuximab, anti-EGFR monoclonal antibody, or interference with its downstream signaling processes can improve tumor treatment with both chemotherapeutic agents and radiation [12,13]. Furthermore, over-expression of the constitutively active variant, EGFRvIII also has been correlated with enhanced radio-resistance [14]. Preclinical data therefore, have generally supported a strong rationale for combining EGFR inhibitors with radiation treatments.
Broadly speaking, two furthest developed strategies for inhibiting EGFR include use of monoclonal antibodies (mAB) against the EGFR receptor and small molecule tyrosine kinase inhibitors (TKIs). Cetuximab and Panitumumab are examples of mABs, and mechanism includes blocking the extracellular binding domain that inhibits dimer formation. TKIs such as gefitinib and erlotinib, target the intracellular tyrosine kinase domain [10]. However, the activity of EGFR is complicated by the signal diversity due to the formation of homo- and heterodimers with other members of the ErbB family and by the specific autophosphorylation patterns within each ErbB family member. This is further compounded by the identification of specific mutations within EGFR that confer sensitivity to certain EGFR inhibitors. The approach of combining an anti-EGFR therapy with cytotoxic agents including radiation in the treatment of patients with cancer remains an area of active investigation [15-20].
1) Cetuximab (Erbitux)Cetuximab is a chimeric mouse anti-EGFR mAB, and is perhaps the most widely studied and developed mAB in this class. While the main study defining the role of cetuximab in conjunction with RT has been based on positive experience in head and neck squamous cell carcinoma patients [18], this agent has also been studied extensively in NSCLC patients.
Of note, recent phase II studies for stage III NSCLC were reported by the Radiation Therapy Oncology Group (RTOG) (RTOG 0324) and Cancer and Leukemia Group B (CALGB) groups [21,22]. In the randomized phase II CALGB study, two novel chemotherapy regimens in combination with concurrent RT was investigated in stage III NSCLC patients. The first group received carboplatin (AUC 5), pemetrexed (500 mg/m2) every 21 days for four cycles with 70 Gy of RT. The second group received the same with addition of cetuximab. Both groups received four cycles of pemetrexed as consolidation therapy. The primary endpoint was 18-month survival with goal of ≥ 55% at which the regimens would be deemed worthy of further study. The carboplatin/pemetrexed/RT arm demonstrated 18-month OS of 58%, and the group with cetuximab, demonstrated 18-month OS of 54%. Combination of thoracic radiation, pemetrexed, carboplatin, with or without cetuximab was demonstrated to be feasible and fairly well tolerated [22].
In the RTOG study, patients were treated with combination of taxol/carboplatin, and cetuximab (225 mg/m2) for 6 weekly cycles, with 6,300 cGy of fractionated RT. All patients received a loading dose (400 mg/m2) of cetuximab 1 week prior to RT, and patients received carboplatin/taxol/cetuximab for 2 additional cycles after completion of radiation treatments. This study demonstrated median survival of 22.7 months, and 2-year OS of 49.3% [21]. Due to the very promising results, cetuximab was included into the RTOG 0617 trial, which is a large randomized phase III study, which also compares two different radiation doses (60 Gy vs. 74 Gy) with concurrent chemotherapy. Current randomization includes chemotherapy plus cetuximab plus RT vs. chemotherapy plus RT, followed by adjuvant chemotherapy vs. chemotherapy plus cetuximab. Results of this study are pending as it is a currently ongoing study.
2) Gefitinib (Iressa)Gefitinib is approved for use as single agent in treatment of chemotherapy refractory NSCLC [10]. It is known to inhibit primarily the EGFR tyrosine kinase, but also has shown some activity for HER-2 kinase albeit at a much lower level [10]. This agents demonstrated promise in phase II studies (Iressa Dose Evaluation in Advanced Lung Cancer [IDEAL]-1, and IDEAL-2) [23,24], but had disappointing results in phase III trials ('Iressa' NSCLC Trials Assessing Combination Treatment [INTACT]-1, and INTACT-2) where it failed to demonstrate additional benefit to standard chemotherapy for advanced lung cancer patients [25,26]. However, subset of patients were noted to have significant response to gefitinib, and subsequently this led to discovery that mutations in the EGFR tyrosine kinase domain may predict for positive response to gefitinib [27,28].
Southwest Oncology Group performed a large phase III trial where stage III NSCLC patients were treated with standard chemo/RT, and after consolidation with docetaxel for 3 cycles, the patients were randomized to maintenance therapy with placebo, or gefitinib 250 mg/day. These were unselected patient population. At interim analysis, patients on the gefitinib maintenance arm had worse OS, and therefore the study was closed [20].
CALGB 30106 [29] is a phase II study designed to evaluate the addition of gefitinib to sequential or concurrent chemoradiotherapy in unresectable NSCLC patients. Patients were categorized into poor risk (≥2 and ≥5% weight loss) and good risk strata (performance status [PS] 0-1, weight loss <5%). All patients received induction chemotherapy with two cycles carboplatin (AUC 6), and paclitaxel (200 mg/m2), plus gefitinib 250 mg from days 1-21. Gefitinib was removed from induction in May 2004 when a randomized phase III trial did not demonstrate a benefit to adding gefitinib with chemotherapy [20]. Poor risk group received 6,600 cGy of External Beam Radiation Therapy (XRT or EBRT) delivered in 33 fractions, with gefitinib 250 mg/day. Good risk stratum patients received same RT and gefitinib, but also received weekly carboplatin (AUC 2), and paclitaxel (50 mg/m2). Consolidation gefitinib was given until progression. For poor risk, progression free survival (PFS) was 13.4 months, and median OS was 19 months. In good risk stratum, PFS was 9.2 months, and median OS was 13 months. Thirteen of 45 tumors had activating EGFR mutations, and 2/13 had T790M mutations. Seven of 45 tumors had KRAS mutations. When analyzed by these molecular phenotypes, no significant difference in outcome was noted. Interestingly, poor risk stratum who received radiation plus gefitinib after induction chemotherapy demonstrated promising survival and PFS outcomes. This will lead to further studies designed to elucidate the role of gefitinib and RT in poor performance status patients with stage III NSCLC. Meanwhile, the good risk stratum patients did not demonstrate a very good outcome, suggesting that addition of gefitinib to chemotherapy/RT regimen may not be beneficial in this patient population. This is consistent with studies of erlotinib and chemoradiation therapy [30].
3) Erlotinib (Tarceva)Erlotinib is also an EGFR TKI that has been approved for use by the US FDA. It also seems to be a fairly potent inhibitor of signaling mediated by mutant EGFRvIII receptor [10]. Findings from two large phase III studies, The Tarceva Lung Cancer Investigation (TALENT) [31] and Tarceva Responses in Conjunction with Paclitaxel and Carboplatin (TRIBUTE) [32] trials demonstrated no significant benefit to the addition of erlotinib to chemotherapy to OS in patients with advanced lung cancer [31,32]. Similar to the gefitinib studies, the lack of demonstrable global benefit to erlotinib pointed to the need for stringent patient selection criteria. In the TRIBUTE study, addition of erlotinib to carboplatin and taxol improved PFS and OS only in the subset of never smokers. National Cancer Institute (NCI) Canada conducted a phase III study of patients with stage IIIB or IV NSCLC, who had failed 1-2 prior chemotherapy regimens. Patients were randomized to receive erlotinib or placebo. OS was improved with erlotinib (6.7 months vs. 4.7 months), and response rate, time to symptomatic progression, PFS were also improved [33]. Since EGFR TKIs appear to be most effective in never smokers and those with EGFR mutations, this question was studied in a phase II study by CALGB group (CALGB 30406). This study evaluated patients who were never/light smokers, and patients were randomized to E (Erbitux) alone, or Erbitux, carboplatin, paclitaxel. This study has only been reported in an abstract form. At median follow up of 30 months, there was no statistically significant difference in PFS with the addition of Erbitux. However, patients with EGFR mutation had significantly improved PFS and OS in both treatment groups compared to patients who did not harbor the EGFR mutation [34].
Potential role for Erlotinib as maintenance therapy for patients with advanced NSCLC after four cycles of platinum-based doublet chemotherapy was reported as a pre-planned analysis of the Sequential Tarceva in Unresectable NSCLC (SATURN) study [35]. In this study, following first line chemotherapy, patients were randomized to erlotinib (150 mg/day) or placebo until progression or unacceptable toxicity. Interestingly, erlotinib maintenance therapy improved PFS in patients who had achieved response (complete response/partial response [PR]/ stable disease [SD]), while OS was significantly improved in patients who had SD after chemotherapy. The latter survival benefit was significant irrespective of tumor histology and/or EGFR mutation status. Erlotinib maintenance also did not negatively impact quality of life [35].
Choong et al. [30] reported on a ping-pong phase I study of Erlotinib with chemoradiotherapy. One group received induction carboplatin and paclitaxel followed by carboplatin/paclitaxel/RT/+erlotinib, while a second group received cisplatin/etoposide/RT+erlotinib followed by taxotere. Erlotinib dose was escalated from 50 mg to 150 mg in three levels in each arm. Median survival in each group was 13.7 months and 10.2 months respectively, with patients who developed rash having an improvement in OS and PFS. This study demonstrates tolerability of such regimen, but with fairly disappointing survival data [30], once again pointing to the need for improved patient selection when using EGFR based treatments.
4) Summary EGFR studiesTremendous efforts have been made to establish the role of combining anti-EGFR targeted compounds with radiation and chemoradiation therapy in many different malignancies. The paradigm for success is the demonstrable benefit of adding mAB cetuximab with RT for patients with locally advanced head and neck cancer. Interestingly, cetuximab is being investigated as a promising agent for combination with chemo/RT in locally advanced NSCLC (RTOG 0617) based on promising data from RTOG 0324 studies [21]. Meanwhile, in locally advanced NSCLC patients, anti-EGFR TKIs have shown no demonstrable benefit in the maintenance/adjuvant setting after chemoradiation treatments. Interestingly, studies have demonstrated that patient selection may be important when designing studies involving EGFR targeted agents. Patients with activating EGFR mutations may need to be categorically selected out from the general wild type (WT) EGFR population based on studies suggesting significant benefit for EGFR targeted therapeutics in the mutated EGFR patients. Meanwhile, other studies involving only chemotherapeutic agents, suggests that EGFR TKIs may demonstrate benefit in EGFR WT tumors for all patients who have had response to first line chemotherapeutics, when the TKI was used for maintenance setting [35]. This suggests that combination of EGFR targeted agents even when used sequentially with a consolidation or maintenance intent, may have differential response effects, for example, after first line chemoradiation treatments, as opposed to first line chemotherapy treatments. Perhaps after chemoradiation treatments, a repeat evaluation of biomarkers may help determine which subset of patients may have benefit from further therapy with anti-EGFR agents. Therefore, the importance of molecular profiling, and patient selection, such as EGFR mutation status, and smoking status in predicting efficacy of anti-EGFR based regimen has become apparent. Future studies involving anti-EGFR treatments in combination with radiation treatments should also incorporate such stringent patient selection criteria to maximize chance of providing benefit for the appropriate patient. Finally, when combining EGFR inhibitors with radiation, the efficacy may vary by tumor type, molecular profile, and also sequencing of the EGFR inhibitor therapy with respect to radiation treatments.
2. Anti-angiogenesis agentsPerhaps no other molecular targets have been investigated for possible tumor treatment strategies more than inhibitors of tumor angiogenesis or agents that act on the tumor vasculature. The formation of tumor vasculature, which is a prerequisite for progressive tumor growth, is initiated and sustained by angiogenic mediators secreted by tumor cells and cells from the surrounding stroma. Inhibitors of angiogenesis have undergone extensive preclinical testing, with some agents moving into clinical trials. Even though there were concerns that an antiangiogenic agent may impair the efficacy of radiotherapy via the enhancement of hypoxia, interestingly, the first clinical trial with a specific inhibitor of angiogenesis, angiostatin, showed a synergistic effect with radiation [36]. A model of normalization of tumor vasculature has been described by Jain [37]. In this model, pro-angiogenic factors from tumors can cause abnormal neovascularization, and inhibition of tumor angiogenesis transiently normalizes the tumor vasculature. This therefore, has the counterintuitive effect of decreasing tumor hypoxia and improving effectiveness of RT. Preclinical studies have been performed in support of this hypothesis, and phase I study of bevacizumab with 5-fluorouracil and RT preoperatively in locally advanced rectal cancer patients also supported this notion [38].
Other mechanisms implicated for consideration of combining radiation treatments with anti-angiogenic agents have been suggested. For example, some proangiogenic factors have been implicated in induction of expression of DNA repair enzymes. Another important mechanism of action includes the targeting of the tumor microenvironment with the combined mode of therapy. A body of preclinical data has suggested that at higher doses of radiation, tumor radio-sensitivity is directly linked to efficacy of endothelial cell death [39]. Therefore, at conventional fractionation dose (~2 Gy per fraction), the relevance of endothelial cell as a target for radiocurability has been raised [40]. However, in preclinical studies, endothelial cell apoptosis may be induced at lower radiation doses by addition of anti-angiogenic drugs or by blocking targets such as phosphatidylinositol 3-kinase/AKT pathways which are activated by ionizing radiation on endothelial cells [41] suggesting additional mechanism of action for improving cytotoxic effects of radiotherapy in combination with such agents. Finally, radio-resistance of some tumors are thought to be in part mediated by presence of cancer stem cells. Interestingly, these cells have been shown to secrete significant amounts of VEGF [42], and raises the question of whether these tumor cells can become a more sensitive target to radiation treatments when combined with anti-angiogenic agents [43].
Similar to the EGFR inhibitors, anti-angiogenic compounds can broadly be classified as monoclonal antibodies directed against anti-angiogenic molecules or their receptors (mAB) or TKIs with narrow or broad spectrum activity against one or more of these receptors. Furthest clinically developed agents which have been FDA approved include the mAB bevacizumab, and TKIs sorafenib, sunitinib, pazopanib. In NSCLC, studies with bevacizumab have been performed with radiation.
1) BevacizumabBevacizumab is a recombinant humanized monoclonal antibody that targets VEGF to inhibit their interaction with the VEGFR. It has a long circulating half life after IV infusion of up to 21 days. Bevacizumab was the first drug to receive approval by the FDA after demonstration of improvement in OS and time to disease progression when used as first line therapy with 5-FU in patients with advanced colorectal cancer. It has since demonstrated efficacy and activity in NSCLC, renal cell carcinoma, glioblastoma, ovarian cancer [40].
Efforts to improve therapeutic ratio by addition of bevacizumab to chemoradiation therapy has been attempted in multiple studies for both small cell lung cancer and NSCLC patients. Unfortunately, these studies have demonstrated that this regimen was associated with incidence of tracheo-esophageal fistula in both small cell and NSCLC cases [44]. Therefore, in the setting of lung cancer, patient selection factors (location of tumor, histology of tumor), and timing of integration of bevacizumab with RT needs to be considered, when designing further studies. Studies are underway aimed at determining potential role and sequencing of bevacizumab, when used with combined modality therapy, in patients with NSCLC.
2) ThalidomideThalidomide is an agent that was originally marketed as a sedative, and was initially taken off market due to concerns of teratogenicity. There has been a resurgence in use and interest in this agent, as it has since then been found to have potent immuno-modulatory effects as well as anti-angiogenic properties [45]. Although its effects are not limited to angiogenesis, there are also suggestions that thalidomide stimulates vessel maturation with implications of vascular normalization which may be an important strategy for anti-neoplastic therapy [46]. Therefore, use of thalidomide with or without RT has been investigated in preclinical and clinical settings [47].
Eastern Cooperative Oncology Group (ECOG) 3598 was a randomized study comparing chemo/RT with or without thalidomide in patients with stage III NSCLC. Patients underwent either carboplatin/taxol with or without thalidomide for 2 cycles, followed by either weekly carboplatin/taxol with RT (60 Gy in 6 weeks) with or without thalidomide, and in the thalidomide group, patients could be treated with adjuvant thalidomide for up to 2 years. There was no difference in PFS or OS with addition of thalidomide [48]. While this may suggest non-efficacy of such combination therapy regimen, another possibility is that such negative studies may point towards a need for a better patient selection when using specific agents.
3) Summary anti-angiogenesis agentsIt is clear from these studies that efficacy and safety of anti-angiogenic agents in combination of radiation and chemoradiation therapy needs to be approached with great caution. It also appears that location of tumor and the agents used in combination with radiation may have a factor in determining the feasibility and tolerability of such regimen. Furthermore, given preclinical evidence that agents such as bevacizumab may improve tumor oxygenation and vascular normalization, it may be worthwhile to consider studies where RT is timed to occur during this window of vascular normalization, which may require more elegant study designs incorporating molecular imaging, and non-concurrent but rather a sequential administration of radiotherapy with these agents.
3. ALK inhibitorsALK fusion protein results in constitutive activation of ALK tyrosine kinase. Soda et al. [49] discovered the fusion of the ALK gene with ELM4-ALK. This ELM4-ALK fusion oncogene has become a very important potential biomarker for patients with NSCLC. The frequency of ALK translocation ranges from 3-7% in unselected NSCLC patients. Furthermore, similar to EGFR mutations, this translocation is seen more frequently in adenocarcinomas, and patients with no or light smoking history. Furthermore, ALK translocations appear to be mutually exclusive with EGFR and KRAS mutations [50]. Several ALK inhibitors have been identified, the furthest developed of which is crizotinib.
1) CrizotinibCrizotinib is an ALK kinase inhibitor, initially designed as inhibitor, but has been found to be clinically effective as ALK inhibitor in NSCLC patients harboring ALK translocations [50]. In a phase I trial of 82 patients selected for ALK translocation (out of over 1,500 patients), an impressive response rate of 57% was noted [51]. Based on this very promising phase I study, this agent has entered phase III studies directly. This agent has received FDA approval for patients with NSCLC with ALK translocation. There are no significant data to suggest a radio-sensitizing or synergistic effect when combined with RT concurrently. However, since agents such as crizotinib targets a very specific population of cancer patients, yielding a potential for high level of response, one strategy being considered is to select patients early on using biomarkers to select patients for clinicial trials involving the use of crizotinib.
Strategies for Effective Clinical Trial Design in the Era of Personalized Medicine for Locally Advanced NSCLCWhile high impact molecular discoveries and effective combined modality treatment realizations have been of significant importance in the field of oncology, another area that has made significant strides and impact on cancer therapeutics is the concept of molecular selection of patients for appropriate therapy. One of the best examples of such is in the field of breast cancer where hormonal therapy, and herceptin treatments are selectively given to patients whose tumors demonstrate appropriate molecular criteria (estrogen receptor positivity, and HER2/NEU over expression) based on the clinical evidence that such patients are most likely to derive benefit from these agents [52]. Another spectacular example of success when drug was applied to appropriately selected patients is the model of the impact of imatinib for treatment of patients with c-kit harboring gastrointestinal stromal tumors [53]. Predictive value of KRAS mutation status for anti-EGFR therapy is established for patients with metastatic colorectal cancer [54]. Meanwhile, abundant studies are in progress and/or have been performed to attempt to determine biomarkers that may provide prognostic or therapeutic information. Multigene assay (Oncotype DX) [55] for example, is already in use in clinical practice for patients with breast cancer. Genomic signatures or biomarkers of response to chemotherapy of tumor, to survival, or to metastatic potential of tumor have been studied [56]. Similarly, while efforts to study and identify biomarkers for radiation response, sensitivity, resistance, or toxicity are being investigated, mature data for use in clinical setting have not yet been accumulated.
1. Incorporating biomarker studies in to the clinical trial designIn NSCLC, as detailed above, studies have demonstrated that patients with EGFR activating mutations derived a striking response rates, and improved PFS to anti-EGFR strategies, even in the metastatic setting, while patients with ALK fusion gene had a remarkable response rate when treated with crizotinib. Elucidation of other molecular targeted agents and biomarker identification to predict response to such agents continues to be an area of active research. What are the current biomarkers available to use, and what is the feasibility of utilizing such biomarkers for rational clinical trial design in the era of personalized medicine? Currently, the leading biomarkers for design of personalized combined modality therapy regimens remain analyzing for EGFR mutations, and ALK translocations. In the EGFR WT and non-rearranged ALK population, studies have yet to identify predictive biomarkers despite several ongoing promising studies, such as that involving use of proteomic analysis [57].
Interestingly, studies spanning Europe, US, and Asia have uniformly indicated that up to 80% of original biopsies could be successfully tested for molecular studies, and fluorescence in situ hydridization, immunohistochemistry, and mutation analysis could be available within 5-7 working days [58,59]. This suggests that biomarker testing in the context of EGFR mutations and ALK translocations should be certainly feasible for incorporation into clinical trial design. Therefore, at minimum, given remarkable findings of these studies, we should be performing ALK and EGFR testing to broadly classify clinical trials into mutation positive, ALK translocation positive, and otherwise negative categories. Furthermore, investigators designing clinical trials utilizing novel agents should actively incorporate biomarker evaluations, to determine which biomarkers may aid in patient selection for their targeted agent.
Another area of active interest would be incorporation of imaging technology into patient selection process. For example when considering vasculature targeting agents, could pretreatment and post-treatment imaging studies of the tumor vasculature help aid in patient selection? With advent of molecular imaging technology, it would be truly remarkable if personalized medicine could involve such non-invasive assessment tools as a surrogate marker of molecular processes, to aid in patient selection criteria.
2. Clinical trial design in the era of personalized medicine using molecularly tailored therapeuticsTherefore, clinical trial design for combined modality therapy in the next decade, will require a level of complexity beyond formulaic addition of two cytotoxic agents to elucidate a synergistic response. It will require an in depth understanding of molecular pathways of the individual cytotoxic agents, including chemotherapy, targeted agents, and ionizing radiation. Such will provide the basis for rationally designed experiments and clinical studies that will most effectively combine chemotherapeutic and biologic agents with RT. Furthermore, carefully designed preclinical studies using appropriate in vivo tumor models will be necessary to help guide the appropriate timing, and mode of RT for a particular cancer type. Thoughtful incorporation of RT technology, and dose fractionation schemes with the appropriate systemic therapy will be required. Finally, incorporation of molecular selection strategies using appropriate biomarkers, or imaging tools as surrogate biomarkers, to design studies aimed at determining what would be the most appropriate and tailored therapeutic strategy for each of the various subset of patients, should be the focus of future clinical trial designs.
As a way of example, when designing clinical trials for stage III NSCLC patients, patients enrolled can be categorized into three subsets based on ALK and EGFR status: i) wildtype EGFR, ii) mutant EGFR, iii) rearranged ALK. Clinical trial design should then be tailored for each subset of patients (Fig. 1). For example, in patients with ALK translocations, randomized trial incorporating crizotinib monotherapy up front as one of the randomization arms, may be a strong consideration, as opposed to treating these patients with chemo/RT regimens upfront. On the other hand, for a subset of patients without mutations, clinical trial design may focus on incorporating novel molecular targets, or systemic agents with standard chemoradiotherapy (+cetuximab) as they are unlikely to benefit from crizotinib or EGFR TKI based treatments.
3. Molecular agents to consider for future combined modality studies for NSCLCWhile there is significant work to be done in terms of designing effective clinical trials incorporating radiation treatments with the currently known molecular determinants (EGFR, ALK), it is also important to realize that there continues to be novel agents coming down the pipeline, some of which show significant promise. As we strive to design combined modality therapy clinical trials aimed at personalizing NSCLC therapy, one focus would be to determine how best to integrate novel compounds and their biomarkers in to these studies. One group of agents receiving significant interest, for example, are the second generation TKIs designed to combat the emerging resistance to a first line EGFR TKI therapy as described below. An all inclusive list of the numerous novel compounds in development would not be possible to discuss within the scope of this article, but some of the interesting new compounds gaining significant attention in the NSCLC field are briefly discussed below.
1) PF299804Several studies, as noted above have demonstrated that patients with NSCLC with EGFR activating mutations are excellent candidates for therapy with EGFR TKIs. Unfortunately, while response to TKIs can be impressive, they are not always durable, as further mutations which can cause resistance to the first line TKI therapy. One such mutation is the T790M (exon 20) mutation which causes treatment resistance to first line TKI drugs [60].
PF299804 is an irreversible TKI which is a pan-Her inhibitor and exhibits activity against both Her2 and Her4. It also inhibits EGFR activating mutations, as well as T790M resistance mutations [61]. A phase I study demonstrated good tolerance to this agent, and 4 patients in this study who had NSCLC with prior treatment with a first line TKI, demonstrated partial response to PF299804 [62]. Patients with K-ras WT NSCLC who have failed erlotinib are being enrolled in a two arm phase II study treating adenocarcinoma and non-adenocarcinoma patients with this agent. Preliminary findings report stable disease in 9/18 evaluable patients in the adenocarcinoma arm, and 1 out of 2 patients in the non-adenocarcinoma arm [63].
2) BIBW2992 (Afatanib)This is also a second generation TKI. Preclinical studies have demonstrated that this agent is a potent irreversible inhibitor of EGFR, and mutated EGFR receptors, including the T790M variant, as well as Her2. Preclinical studies have also suggested additive effects for combination of BIBW2992 with RT, when higher doses of RT was used (20 Gy) in human squamous cell carcinoma cells [64]. Phase I study with BIBW2992 has been reported in patients with advanced solid tumors [65]. BIBW 2992 was generally well-tolerated. The most common adverse effects included diarrhea, nausea, vomiting, rash, and fatigue. Dose-limiting toxicities included grade 3 rash (n=2) and reversible dyspnea secondary to pneumonitis (n=1). Three patients with NSCLC (two with in-frame exon 19 mutation deletions) experienced confirmed PRs sustained for 24, 18, and 34 months, respectively. Several phase II and III trials of this agent are underway in selected NSCLC patients. For example, the LUX-Lung 5 and LUX-Lung 6 trials are studying the role of BIBW2992 in patients who have failed erlotinib/gefinitib, and those with EGFR activating mutations respectively.
3) NeratinibNeratinib is an irreversible pan-ErbB TKI that is being studied as an agent that among other things may potentially overcome resistance due to T790M mutations. Neratinib has been shown to inhibit the growth of T790M mutant cells in vitro in human lung cancer cell lines and in murine cells transfected with sensitizing EGFR mutations [66]. A phase I study demonstrated dose limiting toxicity to be diarrhea, and activity was noted in several heavily pretreated patients with NSCLC [67]. This led to a phase II study recently reported in 2010 in patients with advanced NSCLC. In this study, they examined the role of neratinib in patients who had prior TKI therapy with or without EGFR mutation positivity, as well as in TKI naïve patients with adenocarcinoma and light smoking histories. All patients received daily oral neratinib. The primary end point was objective response rate. Diarrhea was the dose limiting toxicity, with grade 3 incidence of 50% initially, and therefore dose reduction was needed, which improved the grade 3 diarrhea rate to 25%. Of 167 patients treated, the response rate was 3% in patients with mutations, and zero in patients without mutations. TKI naïve patients also had zero response rate. Surprisingly, no patients with known T790M mutation responded. Interestingly, three of four patients with an exon 18 G719X EGFR mutation had a partial response and the fourth had stable disease lasting 40 weeks. The authors concluded that neratinib had a low response rate in patients who were pretreated with TKIs and in TKI naïve patients, with one explanation being possibility of low bioavailability of the drug due to diarrhea. Interestingly, responses were seen in patients with the rare G719X EGFR mutation. This study further highlights the importance of patient selection for future clinical trials [68].
4) AZD6244This is a potent, and selective MEK inhibitor which is being studied in advanced NSCLC patients. Preclinical studies suggest that AZD6244 in vitro exhibited radiosensitizing effects in human cancer cell lines including a NSCLC cell line [69]. Similarly, combining AZD6244 with fractionated radiotherapy in lung and colon carcinoma xenograft models suggested a significant improvement in tumor growth delay [70]. Clinically, a randomized phase II study of patients receiving either 100 mg oral AZD6244 twice daily vs. pemetrexed once every three weeks was reported [71]. Eighty-four patients for whom this was second or third line therapy, were enrolled. Disease progression events were experienced by 28 (70%) and 26 (59%) patients in the AZD6244 and pemetrexed groups, respectively. Median progression-free survival was not statistically different between the AZD6244 and pemetrexed groups. For unselected NSCLC patients with advanced NSCLC, addition of AZD6244 did not seem to confer benefit to pemetrexed. Further development of AZD6244 therefore is also expected to require selection criteria, such as BRAF or Ras mutation positivity in patients with advanced NSCLC.
5) BIBF 1120BIBF 1120 is a potent tyrosine kinase inhibitor which simultaneously inhibits VEGFR 1-3, fibroblast growth factor receptor 1-2, and platelet derived growth factor receptor alpha and beta. A phase I study was performed demonstrating that it was well tolerated to maximum tolerated dose of 250 mg twice/day [72]. In a phase II study of NSCLC patients failing first or second line platinum-based chemotherapy, BIBF 1120 was studied in 73 patients at 250 mg and 150 mg bid doses [73]. The median PFS was 6.9 weeks, and without a significant difference for either dose level. Median OS was 21.9 weeks. Tumor stability was achieved in 46% of patients (59% for ECOG PS 0-1 patients), and one patient at the 250 mg dose level achieved PR [73].
ConclusionBiomarkers, molecular therapeutics, advanced imaging technology, and advances in understanding of effective chemotherapy and radiation treatment integration, have led to an era where personalized medicine for NSCLC is becoming a reality. Studies after studies have pointed towards the need for careful patient selection when designing clinical trials incorporating molecular targeted agents. Effective biomarker development and integration of such into clinical trial design is essential as it becomes more and more clear that locally advanced NSCLC is truly a heterogeneous entity. Tailored, and personalized therapeutic strategies can only be developed when effective biomarkers are identified early on, in order to help improved the odds of using such approaches. There are certainly challenges that we can anticipate along the way towards an era of fully personalized medicine. For example, once numerous biomarkers have been elucidated, how will we decide which biomarkers are most important to test for further clinical trials? Furthermore, how will these studies be financed? In the era of tenuous healthcare finances, once FDA approved, will we have the funds to implement the needed studies, and be able to support payment for all the novel drugs coming out of the pipeline?
Finally, despite significant improvements rendered to stage III NSCLC in the past decades, it is sobering to realize that locally advanced NSCLC remains for the majority of patients, a deadly disease. We must improve our therapeutic development strategy, in order to maximize chance of future success for our patients who are bravely enrolling into clinical trials in hopes of providing valuable insights for future patients afflicted with this dreadful disease. As we embark on this era of personalized medicine for lung cancer therapeutics, we must embrace the opportunities ahead, by designing effective clinical trials, by enrolling patients in to such trials, and encouraging oneself, and others to persevere on with this exciting new paradigm for lung cancer treatment delivery despite the challenges that we will certainly face in the coming era.
References1. Kim DW, Choy H. Combined modality therapy for non-small cell lung cancer, past, present, and future. Lung Cancer. 2003;42(Suppl 2):S35–S40. PMID: 14644534
2. Subramanian J, Madadi AR, Dandona M, Williams K, Morgensztern D, Govindan R. Review of ongoing clinical trials in non-small cell lung cancer: a status report for 2009 from the ClinicalTrials. gov website. J Thorac Oncol. 2010;5:1116–1119. PMID: 20592626
3. Mason KA, Komaki R, Cox JD, Milas L. Biology-based combined-modality radiotherapy: workshop report. Int J Radiat Oncol Biol Phys. 2001;50:1079–1089. PMID: 11429236
4. Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. PMID: 16087941
5. Mendelsohn J, Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997;89:341–343. PMID: 9060954
6. Schmidt-Ullrich RK, Dent P, Grant S, Mikkelsen RB, Valerie K. Signal transduction and cellular radiation responses. Radiat Res. 2000;153:245–257. PMID: 10669545
7. Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–254. PMID: 12909240
8. Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–4673. PMID: 15534360
9. Dickstein BM, Wosikowski K, Bates SE. Increased resistance to cytotoxic agents in ZR75B human breast cancer cells transfected with epidermal growth factor receptor. Mol Cell Endocrinol. 1995;110:205–211. PMID: 7672450
10. Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–4065. PMID: 17827454
11. Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. PMID: 10213503
12. Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6:2166–2174. PMID: 10873065
13. Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–708. PMID: 10690556
14. Lammering G, Hewit TH, Valerie K, Contessa JN, Amorino GP, Dent P, et al. EGFRvIII-mediated radioresistance through a strong cytoprotective response. Oncogene. 2003;22:5545–5553. PMID: 12944901
15. Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. PMID: 10673534
16. Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–3243. PMID: 11432891
17. Tuccillo C, Romano M, Troiani T, Martinelli E, Morgillo F, De Vita F, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor-2 and epidermal growth factor receptor small molecule tyrosine kinase inhibitor, in combination with SC-236, a cyclooxygenase-2 inhibitor. Clin Cancer Res. 2005;11:1268–1276. PMID: 15709198
18. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. PMID: 16467544
19. Harari PM, Huang S. Radiation combined with EGFR signal inhibitors: head and neck cancer focus. Semin Radiat Oncol. 2006;16:38–44. PMID: 16378905
20. Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. PMID: 18378568
21. Blumenschein GR Jr, Paulus R, Curran WJ, Robert F, Fossella F, Werner-Wasik M, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–2318. PMID: 21555682
22. Govindan R, Bogart J, Stinchcombe T, Wang X, Hodgson L, Kratzke R, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol. 2011;29:3120–3125. PMID: 21747084
23. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21:2237–2246. PMID: 12748244
24. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. PMID: 14570950
25. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–784. PMID: 14990632
26. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–794. PMID: 14990633
27. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. PMID: 15118073
28. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. PMID: 15118125
29. Ready N, Jänne PA, Bogart J, Dipetrillo T, Garst J, Graziano S, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol. 2010;5:1382–1390. PMID: 20686428
30. Choong NW, Mauer AM, Haraf DJ, Lester E, Hoffman PC, Kozloff M, et al. Phase I trial of erlotinib-based multimodality therapy for inoperable stage III non-small cell lung cancer. J Thorac Oncol. 2008;3:1003–1011. PMID: 18758303
31. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. PMID: 17442998
32. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. PMID: 16043829
33. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. PMID: 16014882
34. Janne PA, Wang XF, Socinski MA, Crawford J, Capelletti M, Edelman MJ, et al. Randomized phase II trial of erlotinib (E) alone or in combination with carboplatin/paclitaxel (CP) in never or light former smokers with advanced lung adenocarcinoma: CALGB 30406. J Clin Oncol. 2010;28(15s):7503.
35. Coudert B, Ciuleanu T, Park K, Wu YL, Giaccone G, Brugger W, et al. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol. 2012;23:388–394. PMID: 21610154
36. Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. PMID: 9685160
37. Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. PMID: 15637262
38. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. PMID: 14745444
39. Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. PMID: 12750523
40. Mazeron R, Anderson B, Supiot S, Paris F, Deutsch E. Current state of knowledge regarding the use of antiangiogenic agents with radiation therapy. Cancer Treat Rev. 2011;37:476–486. PMID: 21546163
41. Kim DW, Huamani J, Fu A, Hallahan DE. Molecular strategies targeting the host component of cancer to enhance tumor response to radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:38–46. PMID: 16377414
42. Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. PMID: 16912155
43. Zips D, Le K, Yaromina A, Dörfler A, Eicheler W, Zhou X, et al. Triple angiokinase inhibition, tumour hypoxia and radiation response of FaDu human squamous cell carcinomas. Radiother Oncol. 2009;92:405–410. PMID: 19409639
44. Spigel DR, Hainsworth JD, Yardley DA, Raefsky E, Patton J, Peacock N, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol. 2010;28:43–48. PMID: 19901100
45. Raje N, Anderson K. Thalidomide: a revival story. N Engl J Med. 1999;341:1606–1609. PMID: 10564693
46. Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. PMID: 20364125
47. Fanelli M, Sarmiento R, Gattuso D, Carillio G, Capaccetti B, Vacca A, et al. Thalidomide: a new anticancer drug? Expert Opin Investig Drugs. 2003;12:1211–1225.
48. Schiller JH, Dahlberg SE, Mehta M, Johnson DH. A phase III trial of carboplatin, paclitaxel, and thoracic radiation therapy with or without thalidomide in patients with stage III non-small cell carcinoma of the lung (NSCLC): E3598. J Clin Oncol. 2009;27(15s):7503.
49. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. PMID: 17625570
50. Sasaki T, Jänne PA. New strategies for treatment of ALK-rearranged non-small cell lung cancers. Clin Cancer Res. 2011;17:7213–7218. PMID: 22010214
51. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. PMID: 20979469
52. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. PMID: 16236738
53. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. PMID: 19303137
54. Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther. 2010;32:437–453. PMID: 20399983
55. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. PMID: 15591335
56. Kumar R, Amado RG. Predictive genomic biomarkers. Curr Top Microbiol Immunol. 2011;8. 30[Epub]. http://dx.doi.org/10.1007/82_2011_164
57. Amann JM, Lee JW, Roder H, Brahmer J, Gonzalez A, Schiller JH, et al. Genetic and proteomic features associated with survival after treatment with erlotinib in first-line therapy of non-small cell lung cancer in Eastern Cooperative Oncology Group 3503. J Thorac Oncol. 2010;5:169–178. PMID: 20035238
58. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. PMID: 19692684
59. Bunn PA Jr, Hirsch FR, Doebele RC, Camidge DR, Varella-Garcia M, Franklin W. Biomarkers are here to stay for clinical research and standard care. J Thorac Oncol. 2010;5:1113–1115. PMID: 20661083
60. Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. PMID: 19632948
61. Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. PMID: 18089823
62. Jänne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011;17:1131–1139. PMID: 21220471
63. Janne PA, Reckamp K, Koczywas M, Engelman JA, Camidge DR, Rajan A, et al. Efficacy and safety of PF-00299804 (PF299) in patients (pt) with advanced NSCLC after failure of at least one prior chemotherapy regimen and prior treatment with erlotinib (E): a two-arm, phase II trial. J Clin Oncol. 2009;27(15s):8063.
64. Schütze C, Dörfler A, Eicheler W, Zips D, Hering S, Solca F, et al. Combination of EGFR/HER2 tyrosine kinase inhibition by BIBW 2992 and BIBW 2669 with irradiation in FaDu human squamous cell carcinoma. Strahlenther Onkol. 2007;183:256–264. PMID: 17497097
65. Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–3972. PMID: 20679611
66. Wong KK. HKI-272 in non small cell lung cancer. Clin Cancer Res. 2007;13(15 Pt 2):s4593–s4596. PMID: 17671147
67. Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. PMID: 19318484
68. Sequist LV, Besse B, Lynch TJ, Miller VA, Wong KK, Gitlitz B, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–3083. PMID: 20479403
69. Chung EJ, Brown AP, Asano H, Mandler M, Burgan WE, Carter D, et al. In vitro and in vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 kinase. Clin Cancer Res. 2009;15:3050–3057. PMID: 19366835
70. Shannon AM, Telfer BA, Smith PD, Babur M, Logie A, Wilkinson RW, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) enhances the radiation responsiveness of lung and colorectal tumor xenografts. Clin Cancer Res. 2009;15:6619–6629. PMID: 19843666
71. Hainsworth JD, Cebotaru CL, Kanarev V, Ciuleanu TE, Damyanov D, Stella P, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–1636. PMID: 20802351
72. Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C, et al. Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res. 2010;16:311–319. PMID: 20028771
Fig. 1Components of clinical trial design in the era of combined modality therapy and molecular based therapeutics. EGFR, epidermal growth factor receptor; WT, wildtype; ALK, anaplastic lymphoma kinase; XRT, external beam radiation therapy; TKI, tyrosine kinase inhibitor. 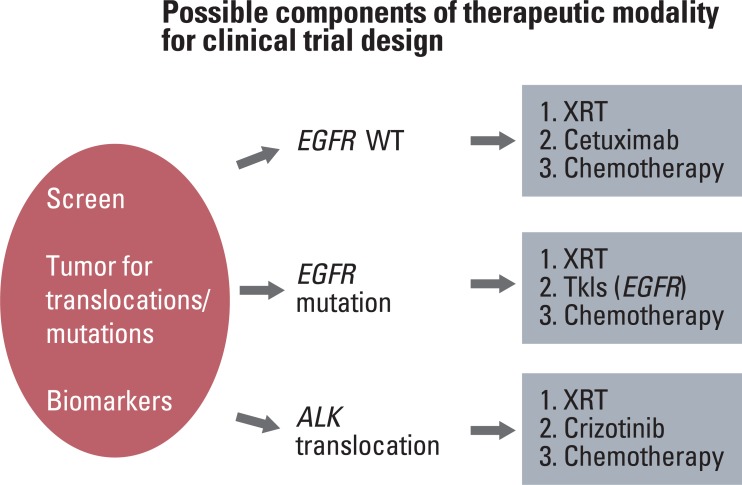
Table 1Clinical trials incorporating molecular compounds with combined chemotherapy and radiation treatments locally advanced non-small cell lung cancer RTOG, Radiation Therapy Oncology Group; EGFR, epidermal growth factor receptor; RT, radiation therapy; OS, overall survival; CALGB, Cancer and Leukemia Group B; SWOG, Southwest Oncology Group; TKI, tyrosine kinase inhibitor; PFS, progression fress survival; VEGF-A, vascular endothelial growth factor A. |
|
|||||||||||||||||||||||||||||||||||||