AbstractPurposeThis study investigated the efficacy and toxicity associated with consolidation chemotherapy using paclitaxel and carboplatin after concurrent chemoradiation (CCR) in cervical cancer patients.
Materials and MethodsFrom a total of 37 patients, 19 with International Federation of Gynecology and Obstetrics (FIGO) stage IB1-IIA cervical cancer (group 1) underwent surgery followed by consolidation chemotherapy after CCR, and 18 with stage IIB-IVA disease (group 2) received consolidation chemotherapy after primary CCR. Three cycles of chemotherapy using paclitaxel (135 mg/m2) and carboplatin (AUC 5.0) were administered every 3 weeks for CCR therapy, and three cycles of consolidation chemotherapy using paclitaxel (175 mg/m2) and carboplatin (AUC 5.0) were used every 3 weeks after CCR.
ResultsThe complete and partial response rates were 77.8% and 22.2% in group 2. Moreover, the 3-year progression-free and overall survival rates were 62.7% and 90.9% in group 1, and 51.9% and 60% in group 2, respectively. The most common grade 3 or 4 hematologic toxicities observed were leukopenia (group 1, 10.5%; group 2, 13.0%) and neutropenia (group 1, 7.0%; group 2, 14.8%), and grade 3 or 4 diarrhea (group 1, 1.8%) and febrile illness (group 2, 1.9%) were the most frequently observed non-hematologic toxicities. When we compared these results with previous reports, consolidation chemotherapy after CCR using paclitaxel and carboplatin revealed a relatively lower complete response rate (77.8% vs. 87-100%, respectively) and shorter progression-free survival (51.9-62.7% vs. 81-86%, respectively) and overall survival (60-90.9% vs. 81-95%, respectively) in spite of similar toxicity findings.
IntroductionThe application of radiation therapy (RT) has been reportedly extended for use in treatment of International Federation of Gynecology and Obstetrics (FIGO) stage IB1 to IVA cervical cancers [1]. In particular, RT is considered an adjuvant therapy after surgery based on histologic intermediate- or high-risk factors, or as a primary therapy in lieu of surgery [2]. Moreover, concurrent chemoradiation (CCR) has been established as being more effective than RT alone because chemotherapy has been shown to increase the sensitivity of tumor cells to radiation and to control both local and systemic disease manifestations [3].
Since 2000, cisplatin-based CCR has been found to be the most effective treatment for patients with high-risk early-stage or locally advanced cervical cancer [4], and various types of single agent or combination chemotherapies including cisplatin, hydroxyurea, ifosfamide and 5-flurouracil (5-FU) have been introduced in order to improve clinical outcomes in patients [4-6]. Furthermore, consolidation chemotherapy using epirubicin, 5-FU and cisplatin after CCR has been reported to enhance local control and promote eradication of distant micro-metastases in locally advanced cervical cancer [7-9].
Combination therapy using paclitaxel and carboplatin is the standard chemotherapeutic regimen producing acceptable toxicity in treating patients with epithelial ovarian cancer [10,11]. But there is a lack of acceptable efficacy and toxicity evidence supporting their use in CCR treatment for patients with cervical cancer, and relevant clinical trials are ongoing [12,13]. We reported a 3-year progression-free survival (PFS) of 88.2% and an overall survival (OS) of 97.3% in patients with high-risk early-stage cervical cancer, and a 3-year PFS of 75% and an OS of 86% with a complete response (CR) of 70% and partial response (PR) of 10% in patients with locally advanced cervical cancer when using paclitaxel and carboplatin for CCR [14,15].
Thus, we hypothesized that consolidation chemotherapy using paclitaxel and carboplatin would improve tumor response and survival in patients. We therefore performed a phase II clinical trial of consolidation chemotherapy using paclitaxel and carboplatin after adjuvant CCR for subjects with high-risk early-stage cervical cancer who had undergone primary surgery, or after primary CCR for those with locally-advanced disease.
Materials and Methods1. Study designAfter designing this clinical trial, we registered it in advance with the US National Institutes of Health (NIH) (registration no., NCT-00591656 and -00592059). The current study took place at Seoul National University Hospital (SNUH) and the clinical protocol was approved by the SNUH Institutional Review Board. Patients were enrolled after providing their written informed consent.
The current study was performed with two groups in order to evaluate the efficacy and toxicity of consolidation chemotherapy using paclitaxel and carboplatin following CCR as follows: group 1 consisted of patients with stage IB1 to IIA cervical cancer who underwent type II (n=17) or III (n=2) hysterectomy with pelvic or para-aortic lymphadenectomy, and received 3 cycles of consolidation chemotherapy after adjuvant CCR based on histologic results such as one or more high-risk factors (positive resection margin, parametrial invasion or lymph node metastasis); group 2 included patients with locally advanced cervical cancer (stage IIB to IVA) who received primary CCR followed by 3 cycles of consolidation chemotherapy.
The eligibility criteria were as follows: age > 20 years, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1, life expectancy > 6 months, and normal hematologic, renal and liver function. We excluded patients with a history of other malignancies, prior chemotherapy or RT, or underlying diseases which might influence clinical outcomes.
2. TreatmentExternal-beam radiotherapy (EBRT) with concurrent chemotherapy was performed with patients in both groups. EBRT was delivered to the whole pelvis using 6 or 10 megavoltage photons through either the parallel-opposed (antero-posterior/postero-anterior) ports, or the four-field box technique. The upper and lower borders of the EBRT ports were at the L5-S1 junction and at least 2 cm below the vault of the vaginal cuff or the gross tumor, respectively. The lateral edges were set to 1.5 cm lateral to the bony pelvis. The anterior and posterior borders were the anterior aspect of the symphysis pubis and the S2-S3 junction, respectively. The median radiation dose of 50.4 Gy (range, 50.0 to 61.2 Gy) was delivered in 1.8-2.0 Gy fractions once daily, 5 days per week.
If there were gross para-aortic lesions over the T12-L1 junction, the field of the EBRT irradiation was continuous with extension to the T12-L1 junction or higher. The median radiation doses delivered to para-aortic lesions were 45 Gy (range, 45.0 to 61.2 Gy) in group 1 patients, with histological confirmation of para-aortic lymph node metastasis, and 60 Gy (range, 45.0 to 63.0 Gy) in all group 2 patients. Intracavitary irradiation (ICR) was administered to all patients in group 2. ICR was delivered using the Fletcher-Suit unit with 137Cs sources with a median dose of 31.4 Gy (range, 22.0 to 34.6 Gy).
Chemotherapy consisted of three cycles of paclitaxel (135 mg/m2) and carboplatin (AUC 5.0, Calvert's formula) every 3 weeks during CCR. After CCR, three cycles of consolidation chemotherapy using paclitaxel (175 mg/m2) and carboplatin (AUC 5.0, Calvert's formula) were administered every 3 weeks. When chemotherapy was delayed due to the appearance of grade 3 or 4 toxicity, we re-evaluated the toxicity after one week. And if resolved, we administered a 75% dose of paclitaxel and carboplatin. Although our study design disqualified patients if chemotherapy was delayed more than 3 weeks, no patients were excluded.
3. Response, toxicity, recurrence and survivalTumor response and disease recurrence were investigated by physical examination and imaging studies including chest X-ray, computed tomography and positron emission tomography according to response evaluation in solid tumors (RECIST) criteria [16]. We checked tumor response one month after consolidation chemotherapy during this clinical trial.
Toxicity was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) ver. 3.0. PFS and OS were defined as the time elapsed from the date of diagnosis to the date of clinically proven recurrence, and the time elapsed from the date of diagnosis to the date of cancer-related death or the end of the current study, respectively.
4. Statistical analysisPatient characteristics, tumor response and toxicity were evaluated using descriptive summary statistics. PFS and OS were calculated by the Kaplan-Meier method. The 95% confidential intervals (CIs) for the estimated response and survival rate were calculated using binominal distribution. Statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL). A p-value <0.05 was considered statistically significant.
Results1. Patient characteristicsA total of 37 patients were enrolled between November 2006 and December 2009. Of the total, 19 (group 1) underwent primary surgery plus adjuvant CCR followed by 3 cycles of consolidation chemotherapy, whereas 18 patients (group 2) received primary CCR followed by 3 cycles of consolidation chemotherapy using paclitaxel and carboplatin. Clinical characteristics of the study participants are depicted in Table 1. The median age of all patients was 50 years (range, 24 to 74 years) and the median duration of follow-up was 35.3 months (range, 10.2 to 50.7 months). Of the total patients, 32 (86.5%) were histologically diagnosed with squamous cell carcinoma, 3 (8.1%) had adenocarcinoma, 1 (2.7%) had adenosquamous carcinoma and 1 (2.7%) had mixed carcinoma consisting of squamous cell carcinoma and adenocarcinoma. All patients completed their planned CCR and then received 3 cycles of consolidation chemotherapy.
2. Consolidation chemotherapy after post-surgery adjuvant CCR1) ToxicityIn group 1, the most common grade 3 or 4 hematologic toxicities observed were leukopenia (total, 10.5%; CCR, 7.0%; consolidation chemotherapy, 14.0%) and neutropenia (total, 7.0%; CCR, 3.5%; consolidation chemotherapy, 10.5%). Six patients (31.6%) received a 75% dose of paclitaxel and carboplatin with a mean one-week delay. However, all patients recovered by conservative management or the administration of granulocyte colony-stimulating factor (G-CSF) without life-threatening complications. Furthermore, the most frequent grade 3 or 4 non-hematologic toxicity observed was diarrhea (total, 1.8%; CCR, 3.5%; consolidation chemotherapy, 0%), and one patient (5.3%) presented grade 3 genitourinary toxicity as cystitis with hematuria, a late RT-related toxicity (Table 2).
2) Recurrence and survivalSubsequent to consolidation chemotherapy after post-surgery CCR, 7 patients (36.8%) showed disease recurrence at lymph nodes (n=3), bone (n=1), lung (n=1), vaginal vault (n=1) and peritoneum seeding with lymph nodes (n=1). The 3-year PFS and OS rates were 62.7% (95% CI, 26.7 to 40.7) and 90.9% (95% CI, 42.3 to 47.1), respectively (Fig. 1).
3. Consolidation chemotherapy after primary CCR1) ToxicityIn group 2, the most frequent grade 3 or 4 hematologic toxicities observed were neutropenia (total, 14.8%; CCR, 16.7%; consolidation chemotherapy, 13.0%) and leukopenia (total, 13.0%; CCR, 16.7%; consolidation chemotherapy, 9.2%). Eight patients (44.4%) received a 75% dose of paclitaxel and carboplatin with a mean two-week delay. These toxicities were improved by supportive care or the administration of G-CSF without any treatment-related deaths. The most common grade 3 or 4 non-hematologic toxicity observed was febrile illness (total, 1.9%; CCR, 3.7%; consolidation chemotherapy, 0%) and one patient (5.6%) showed grade 4 gastrointestinal toxicity consisting of proctitis with perforation as a late RT-related toxicity (Table 2).
2) Response, recurrence and survivalSubsequent to consolidation chemotherapy after CCR, 14 (77.8%) and 4 patients (22.2%) demonstrated CR and PR, whereas 8 patients (44.4%) showed disease recurrence at lymph nodes (n=5), bone (n=1), peritoneum (n=1) and cervix with lymph nodes (n=1). The 3-year PFS and OS rates were 51.9% (95% CI, 25.0 to 42.5) and 60% (95% CI, 31.6 to 46.5), respectively (Fig. 1).
DiscussionThe aim of this phase II clinical trial was to evaluate the efficacy and toxicity of 3 cycles of consolidation chemotherapy after CCR using paclitaxel and carboplatin in patients with stage IB1 to IIA cervical cancer who had undergone primary surgery, or those with stage IIB to IVA cervical cancer. Chemotherapy regimens using paclitaxel and carboplatin have been focused on by previous studies, whereby taxanes and platinum agents have been shown to have different molecular targets with synergistic anti-cancer effects and acceptable toxicities [14,15,17]. In spite of the increasing attention placed on the use of paclitaxel and carboplatin in treating gynecologic cancers, there are no relevant clinical trials demonstrating the efficacy and undesirable toxicities of consolidation chemotherapy or CCR using these drugs.
In the current study, we found that consolidation chemotherapy after CCR using paclitaxel and carboplatin failed to improve PFS and OS as compared to CCR-only in patients with high-risk early-stage cervical cancer who underwent surgical treatment (3-year PFS, 62.7% vs. 88.2%, respectively; 3-year OS, 90.9% vs. 97.3%, respectively) [14]. Although tumor response was similar between the current trial and our previous study for those patients with locally advanced cervical cancer (CR, 77.8% vs. 70%, respectively; PR, 22.2% vs. 10%, respectively), consolidation chemotherapy using paclitaxel and carboplatin failed to increase 3-year PFS and OS as compared to CCR-only (3-year PFS, 51.9% vs. 75%, respectively; 3-year OS, 60% vs. 86%, respectively) [15].
In order to identify the efficacy and toxicity results of the use of paclitaxel and carboplatin in consolidation chemotherapy after CCR in treating cervical cancer, we compared our current results with those of relevant previous reports where the efficacy and toxicity of consolidation chemotherapy using platinum-based drugs after CCR had been investigated (Table 3) [7-9,18,19]. Although the types of ICRs were different between all the studies evaluated, a previous meta-analysis had concluded there was no difference in efficacy between the high dose rate and low dose rate ICRs [20]. Our assessment found that the observed hematologic toxicities were similar between our current study and the previous relevant studies. The previous studies showed 2.9-36% (per patient) and 7.5-10.9% (per cycle) of grade 3 or 4 leukopenia or neutropenia, whereas our current study demonstrated 7.0-14.8% (per cycle). Nevertheless, the CR rate was lower in our current study (77.8%) than as observed in previous studies (87-100%) in patients with locally advanced cervical cancer, and our current study revealed a shorter PFS (51.9-62.7% vs. 81-86%, respectively) and OS (60-90.9% vs.81-95%, respectively) in spite of a relatively small number of patients with stage IIIA to IVA cervical cancer as compared with previous studies (33.3% vs. 22.2-55.2%, respectively). These findings suggested that the use of paclitaxel and carboplatin for consolidation chemotherapy after CCR may not be effective in improving clinical outcomes in patients with locally advanced cervical cancer as compared to other platinum-based chemotherapeutic regimens, in spite of there being no difference in observed toxicities.
The current study had two limitations. First, despite a few retrospective studies where its efficacy was demonstrated, the efficacy of CCR using paclitaxel and carboplatin has not been established in clinical trials for high-risk early-stage or locally advanced cervical cancer. Second, the number of enrolled patients was small which may have resulted in study data bias. In spite of these limitations, our current study indicated that, due to relatively low efficacy, consolidation chemotherapy using paclitaxel and carboplatin after CCR is not recommendable for patients with high-risk early-stage or locally advanced cervical cancer.
However, the merits of the use of paclitaxel with carboplatin include reduced nephrotoxicity, requiring no hydration, relatively brief administration time in the outpatient setting, and a lower rate of nausea and vomiting, which increases the compliance to chemotherapy dosing [12]. As paclitaxel and cisplatin have been shown to be superior to other combination chemotherapies in recurrent or persistent cervical cancer [21], carboplatin with paclitaxel, instead of cisplatin, is also expected to produce a promising and feasible combination for consolidation chemotherapy after CCR in treating cases of locally advanced cervical cancer [12,22]. Furthermore, chemotherapy using paclitaxel (40 mg/m2) and carboplatin (AUC 2.0, Calvert's formula) once a week has been shown to increase tumor response (CR, 90.1%; PFS, 90%) with acceptable toxicity, suggesting that consolidation chemotherapy using weekly paclitaxel and carboplatin after CCR may show high efficacy and low toxicity in the treatment of cervical cancer [23].
ConclusionConsolidation chemotherapy using dose-dense combination of paclitaxel and carboplatin after CCR every 3 weeks is not feasible to treat high-risk early-stage or locally advanced cervical cancer because of low efficacy. However, a promising combination of weekly paclitaxel and carboplatin is expected to be worthy of the need for additional clinical trials to approve high efficacy and low toxicity.
References1. Suh DH, Kim JW, Kim K, Kang SB. Major clinical research advances in gynecologic cancer in 2010. J Gynecol Oncol. 2010;21:209–218. PMID: 21278881
2. Cervical cancer clinical practice guidelines in oncology (v.I.2011) [Internet]. National Comprehensive Cancer Network. cited 2010 Dec 24Fort Washington, PA: National Comprehensive Cancer Network; Available from: http://www.Nccn.Org
3. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. PMID: 11564482
4. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. PMID: 10202165
5. Petera J, Odrázka K, Frgala T, Spacek J. External beam radiotherapy and high-dose brachytherapy combined with cisplatin and paclitaxel in patients with advanced cervical carcinoma. Gynecol Oncol. 2005;99:334–338. PMID: 16023181
6. Kim YS, Shin SS, Nam JH, Kim YT, Kim YM, Kim JH, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol. 2008;108:195–200. PMID: 17963825
7. Vrdoljak E, Prskalo T, Omrcen T, Situm K, Boraska T, Frleta Ilić N, et al. Concomitant chemobrachyradiotherapy with ifosfamide and cisplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: results of a phase II study. Int J Radiat Oncol Biol Phys. 2005;61:824–829. PMID: 15708262
8. Chung YL, Jian JJ, Cheng SH, Hsieh CI, Tan TD, Chang HJ, et al. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: a phase I/II study. Gynecol Oncol. 2005;97:126–135. PMID: 15790448
9. Choi CH, Lee JW, Kim TJ, Kim WY, Nam HR, Kim BG, et al. Phase II study of consolidation chemotherapy after concurrent chemoradiation in cervical cancer: preliminary results. Int J Radiat Oncol Biol Phys. 2007;68:817–822. PMID: 17379437
10. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. PMID: 12860964
11. Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. PMID: 10793106
12. Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Jpn J Clin Oncol. 2010;40:90–93. PMID: 19825815
13. Korean Gynecologic Oncology GroupA phase II trial of radiation therapy with concurrent paclitaxel/carboplatin chemotherapy in high-risk cervical cancer patients after radical hysterectomy [Internet]. ClinicalTrials.gov. cited 2012 Jan 18Available from: http://clinicaltrialgov/ct2/show/NCT00340184?term=paclitaxel+and+carboplatin+and+concurrent+chemoradiation+and+cervical+cancer&rank=2
14. Kim K, Chie EK, Wu HG, Ha SW, Kim JS, Kim IA, et al. Efficacy of paclitaxel and carboplatin as a regimen for postoperative concurrent chemoradiotherapy of high risk uterine cervix cancer. Gynecol Oncol. 2006;101:398–402. PMID: 16330087
15. Lee MY, Wu HG, Kim K, Ha SW, Kim JS, Kim IA, et al. Concurrent radiotherapy with paclitaxel/carboplatin chemotherapy as a definitive treatment for squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2007;104:95–99. PMID: 16996117
16. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of CancerNational Cancer Institute of the United StatesNational Cancer Institute of CanadaNew guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. PMID: 10655437
17. de Vos FY, Bos AM, Gietema JA, Pras E, Van der Zee AG, de Vries EG, et al. Paclitaxel and carboplatin concurrent with radiotherapy for primary cervical cancer. Anticancer Res. 2004;24:345–348. PMID: 15015619
18. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. PMID: 10764420
19. Zhang MQ, Liu SP, Wang XE. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: preliminary results of a phase II study. Int J Radiat Oncol Biol Phys. 2010;78:821–827. PMID: 20207507
20. Wang X, Liu R, Ma B, Yang K, Tian J, Jiang L, et al. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev. 2010;(7):CD007563PMID: 20614461
21. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. PMID: 19720909
22. Kitagawa R, Katsumata N, Yamanaka Y, Ando M, Fujiwara Y, Kasamatsu T. Phase II trial of paclitaxel and carboplatin in patients with recurrent or metastatic cervical carcinoma. J Clin Oncol. 2004;22(14S):5048.
23. Higgins R, Bussey M, Naumann W, Hall J, Tait D, Haake M. Concurrent carboplatin and paclitaxel with pelvic radiation therapy in the primary treatment of cervical cancer. Am J Obstet Gynecol. 2007;197:205.e1–205.e5. PMID: 17689652
Fig. 1Efficacy of paclitaxel and carboplatin as consolidation chemotherapy after concurrent chemoradiation (CCR) in patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB1-IIA (group 1) and stage IIB-IVA (group 2) cervical cancer; (A) progression-free survival and (B) overall survival curves for 19 patients who underwent primary surgery followed by three cycles of consolidation chemotheray after CCR (group 1) and 18 patients who received primary CCR followed by three cycles of consolidation chemotherapy (group 2). 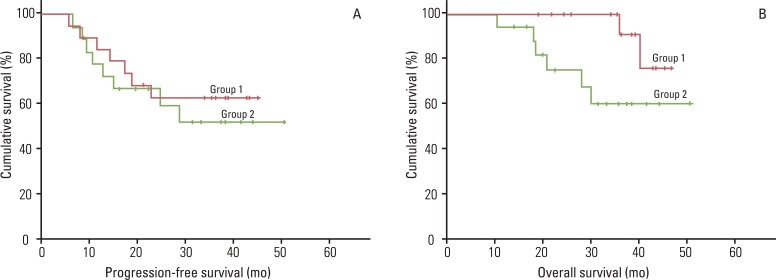
Table 1Patient characteristics (n=37)
Table 2Grade 3 or 4 acute and late toxicities Table 3Summary of relevant studies for investigating the efficacy and toxicity of consolidation chemotherapy after CCR in cervical cancer
CCR, concurrent chemoradiation; FIGO, International Federation of Gynecology and Obstetrics; RT, radiation therapy; CR, complete response; SC, squamous cell carcinoma; AC, adenocarcinoma; ASC, adenosquamous carcinoma; EBRT, external beam radiation therapy; F, 5-fluorouracil; P, cisplatin; PFS, progression-free survival; OS, overall survival; I, ifosfamide; LDR, low dose rate intracavitary irradiation; R-V, rectovaginal; V-V, vesicovaginal; HDR, high dose rate intracavitary brachytherapy; T, paclitaxel; N, nedaplatin; C, carboplatin. a)Number of events per patients, b)Number of events per cycles of chemotherapy, c)The current study. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||