AbstractPurposeIncreasing experimental evidence indicates that abnormal expression of c-kit may be implicated in the pathogenesis of a variety of solid tumors. It has been reported that over 70% of small cell lung cancer (SCLC) contain the c-kit receptor. In the present study, a c-kit analysis has been extended to non-small cell lung cancer (NSCLC). The expressions of p53, vascular endothelial growth factor (VEGF) and cd34, in addition to c-kit, were evaluated to investigate the correlations between these proteins and to determine their potential relationships with the clinicopathological data.
Materials and MethodsParaffin-embedded tumor sections, obtained from 147 patients with NSCLC, were immunohistochemically investigated using anti-c-kit, anti-p53, anti-VEGF and anti-cd34 antibodies.
Resultsc-kit was expressed in 40 (27%) of the tumors examined: 27% of the adenocarcinomas, 27% of the squamous cell carcinomas and 29% of the undifferentiated carcinomas. p53 and VEG F immunoreactivities were present in 107 (73%) and 110 (75%) carcinomas, respectively. Anti-cd34 was negative in all samples. No associations were established among these proteins. The c-kit, however, showed a strong correlation with the T factor: T1 (n=11), 0%; T2 (n=49), 16% and T3 (n=87), 37% (p=.006).
ConclusionIt is suggested that in NSCLC c-kit is expressed relatively frequently and may become a therapeutic target for the patients with inoperable or recurrent c-kit positive tumors. The alterations in p53 probably constitute an early event, whereas the activated c-kit may contribute to tumor progression.
INTRODUCTIONThe kit receptor is a 145~165 kd transmembrane protein that exhibits protein tyrosine kinase activity (1). Its ligand, stem cell factor (SCF), is an early hematopoietic growth factor that, in conjunction with other hematopoietic growth factors, supports the proliferation and differentiation of multiple hematopoietic cell lineages, and has an effect on the control of gametogenesis and melanogenesis through the interaction between c-kit and SCF. Binding of SCF to the kit receptor results in tyrosine autophosphorylation, leading to the activation of its kinase activity, which ultimately induces the complex biological responses involved in the normal cellular physiology as well as in tumorigenesis (2). Abnormal expression of c-kit has been implicated in the pathogenesis of myeloid leukemias and various solid human malignancies, such as gastrointestinal stromal tumors, melanomas, glioblastomas, germ cell tumors, and breast and small cell lung cancers (SCLC) (3~10). SCLC is a distinct clinicopathological entity among lung cancers, with a very aggressive clinical course and neuroendocrine properties. Because of the relatively high percentage of c-kit expression in SCLC (8,9), their rapid progression may be explained by the stimulation of tumor cell growth induced by growth factors, including the ligand for the c-kit product.
In the present study, a c-kit analysis was extended to non-small cell lung cancer (NSCLC) to determine the frequency of its expression and any potential relationships between c-kit expression, pathological factors and clinical outcomes. All the cases were further immunohistochemically analyzed using anti-cd34, anti-p53 and anti-vascular endothelial growth factor (VEGF) antibodies.
MATERIALS AND METHODS1) PatientsTumor tissue samples were obtained from the primary tumors of 147 NSCLC patients, whose follow-up data were available. All patients had received no prior therapy and were surgically treated in this institute between January 1, 2000 and December 31, 2002. Tumors were classified according to the WHO classifications (11), and staged according to the tumor-node-metastasis staging system recommended by the American Joint Committee on Cancer (12). Clinical information was obtained through a computerized prospective database of the tumor registry. The survival times of the patients were calculated from the date of surgery. The follow-up was conducted until August 2003, for a mean of 78 weeks (19 months). Eighty-two patients were alive at the time of this study, while 36 died and 29 were lost to follow-up.
2) Immunohistochemical stainingTissue samples were fixed in 10 percent buffered formalin. After routine embedding, the final diagnosis was made by light microscopy. Immunohistochemical staining was performed on the representative sections of archival, formalin-fixed, paraffin-embedded material using a sensitive peroxidase-streptavidin method, as described previously (13), for the detection of the following antigens: c-kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), cd34 (Zymed Laboratories Inc., San Francisco, CA), p53 (Zymed) and VEGF (Santa Cruz). Briefly, 5µm thick sections were mounted on poly-L-lysine-coated slides, deparaffinized in xylene and rehydrated in graded alcohols and water. The endogenous peroxidase was blocked by soaking in 3% H2O2 at 45℃ for 4 min. Antigen retrieval was performed by immersing the slides in a citrate buffer (2.1 g/l, pH 6.0) and then heating in a microwave oven for 15 min. The slides were treated with a protein blocking reagent before incubation, overnight, at 4℃, with primary antibodies at a 1 : 50 dilution, as recommended by the supplier. After extensive washing, the sections were incubated at room temperature for 10 min with biotinylated anti-mouse immunoglobulin antibodies (Zymed), at a 1 : 20 dilution, and subsequently with streptavidin-biotin peroxidase complexes at a 1 : 25 dilution. The color was developed by incubating the slides for 20 min with 3, 3'-diaminobenzidine tetrahydrochloride (Zymed). Counterstaining was performed using Mayer's hematoxylin.
All series included positive and negative controls. A gastrointestinal stromal tumor specimen, with known c-kit positivity, was used as the positive control for c-kit expression. Other positive controls included; capillaries of normal lung tissue for cd34, breast carcinoma for p53 and bronchial epithelium for VEGF. As negative controls of the immunohistochemistry technique, the primary antibody was omitted. For c-kit and cd34, immunohistochemical findings were scored positive when ≥30% of the neoplastic tissue was immunoreactive along the cell-surface membrane and/or in the cytoplasm. Tumor cells showing a nuclear staining pattern were interpreted as positive for p53. VEGF immunostaining was considered positive if cytoplasmic staining was clearly present in ≥30% of the carcinoma cells.
RESULTSThe clinicopathological data are shown in Table 1, along with the immunohistochemical status. Of the 147 patients, there were 106 men (72%) and 41 women (28%). The patients ages ranged from 33 to 87 years (median 63 years): 32 were less than 58 years of age and 115 were 58 years or older. Patients less than 58 years of age had longer survivals, but this was not significant. As for the tumor type, 63 had squamous cell carcinomas, 60 adenocarcinomas and 24 undifferentiated carcinomas. There were 8, 13 and 126 patients with stages I, II and III tumors, respectively. The stage had an effect on the survival (p=.016); histological type, tumor extent and lymph node status exhibited no effects on the survival.
All the samples were investigated for the expressions of c-kit, cd-34, p53 and VEGF. The negative and positive controls showed the expected results. c-kit expression was identified in 40 cases (27%) (Fig. 1); 27, 27 and 29% of the squamous cell carcinomas, adenocarcinomas and undifferentiated carcinomas, respectively. Of the 60 tumors with either T1 or T2, positive immunostaining for c-kit was observed in 8 (13%), while it was expressed in 32 (37%) of the 87 T3 tumors (p=.006): T1 (n=11), 0%; T2 (n=49), 16% and T3 (n=87), 37%. The majority of c-kit-positive cases were lymph node-positive or a stage III disease, but with no statistical significance. cd-34 was detected in none of the samples. p53 was overexpressed in 107 (73%) samples (Fig. 2). Forty-eight of the 60 (80%) T1 or T2 tumors had detectable p53 protein compared with 59 of the 87 (68%) T3 specimens: this difference approached statistical significance for a negative correlation (p=.053). Positive immunoreactivity for VEGF was observed in 110 cases (75%) (Fig. 3). As with the c-kit expression, the majority of p53- or VEGF-positive samples had a tendency toward lymph node involvement and a higher stage, but no statistical significance was found. No association was established between the expressions of c-kit, cd-34, p53 and VEGF. Fig. 4 shows the survival curves (Kaplan-Meier estimates) of patients according to the clinicopathological parameters. The mean survival time had no significant correlation with the molecular marker status.
DISCUSSIONThe expressions of c-kit, cd-34, p53 and VEGF were analyzed in 147 NSCLC, and evaluated for possible correlations with the patients' clinicopathological parameters (age, gender, and tumor extent, presence of lymph node metastases, tumor stage and patient survival). The major findings of our study were as follows: (1) c-kit was expressed in a significant fraction of NSCLC, independent of histological types; (2) p53 and VEGF were present in the majority of the samples, while cd-34 was lacking and (3) c-kit showed a strong correlation with the T factor.
The understanding of cancer pathogenesis is rapidly growing, with mounting evidence indicating the involvement of several molecular genetic markers in the initiation and/or progression of the disease. The interactions of the molecular genetic changes are complex; however, some may be important targets for the development of specific inhibitors, serving as potential chemotherapeutic agents. The c-kit proto-oncogene appears to be involved, via the SCF/c-kit pathway, in the tumorigenesis of human malignancies, including blood-cell malignancies, as well as gastrointestinal tumors, neuroblastomas, breast carcinomas, testicular germ cell tumors and gynecological tumors (3~7). The fact that kit activation is likely to contribute to the development of these tumors has led to efforts to block kit-mediated mitogen signaling. Several investigations have demonstrated the effectiveness of tyrosine kinase inhibitors on tumors with kit expression (7,10,14). Accordingly, kit-inhibitors and their derivatives may play roles in the treatment of tumors where kit activation occurs. In lung tumors, co-expression of both the kit receptor and its ligand, SCF, has been reported in at least 70% of SCLC cell lines and tumor specimens (8,9). STI-571, a tyrosine kinase inhibitor, was shown to be useful in a clinical trial for patients with SCLC (10,15). Since SCLC comprises only 20 to 25% of all lung cancers, it is important to establish the extent of receptor expression in NSCLC. The literature; however, contains very limited data regarding the kit status in this group of tumors. Pietsch et al. (16) demonstrated 25% (26/106) c-kit immunoreactivity, which was consistent with our results (27%). However, in their study, the incidence varied according to the histological subtype; 30% of adenocarcinomas, 15% of squamous cell carcinomas and 40% of large-cell undifferentiated carcinomas. Although this may reflect a dissimilar significance in different NSCLC subtypes, our study demonstrated no difference in the frequency according to the histological type. Differences in tumor differentiation or analytical procedures, and tumor heterogeneity, may have contributed to this discrepancy.
Despite the rapidly expanding literature on the biochemical and physiological properties of c-kit protein, few data are available on NSCLC with regard to its potential relationship to the clinicopathological variables. Our data revealed a significant correlation between the c-kit activity and the tumor extent. Thus, it is likely that c-kit induces malignant progression in these tumors. Further studies, including a large number of tumors, will be necessary to find its application as a potential marker in the progression of NSCLC.
p53 is the most common and important tumor suppressor gene (17,18). Aberrant expression of p53 has been reported in various malignancies, including lung cancer. Studies have found p53 alterations in 40~80% of NSCLC, with more frequent mutations in tumors of squamous histology (19,20). In the present study, the overall p53 overexpression rate was 72%, which was independent of the histological types. The prognostic significance of altered p53 is still controversial; abnormal p53 has been described as an independent prognostic factor for a favorable outcome in some studies, but unfavorable in others (21,22). Our data revealed significance of p53 expression on the prognosis. However, it was most frequent in T1 tumors (91%) compared to T2 (78%) and T3 (68%) tumors. The expressions in T1 or T2 versus T3 tumors approached statistical significance for a negative relationship, with a p-value of 0.053. Along with the previous findings of mutations in p53 in squamous dysplasia and atypical adenomatous hyperplasia associated with NSCLC (23), this may suggest that, unlike c-kit, p53 plays no critical role in tumor progression, but is involved in the early stage of the development of lung cancer. The widespread loss of heterozygosity for p53 has been detected in stage I recurrent and non-recurrent tumors (19), supporting that p53 alteration may occur early, and by its self may not be sufficient for tumorigenesis, but create genetic instability that allow the other critical mutations to develop.
Several recent studies have indicated that p53 might be involved in angiogenesis, which is an essential process in the development and progression of malignant tumors, including NSCLC. VEGF is one of the most potent angiogenic factors, and seems to play an important role in the proliferation and migration of endothelial cells, providing nourishment to the growing tumors. In NSCLC, VEGF-positivity varied from 61 to 92.3% (24), which was in agreement with our result of 75% VEGF immunoreactivity. VEGF expression has been found to be a significant independent prognostic factor for the survival of patients with NSCLC. However, Baillie et al. (25) failed to establish any correlation in NSCLC. In the present study, the mean survival time did not correlate with the VEGF status. Although T1 and T2 tumors had lower percentages of VEGF-positivity than T3 tumors (62 vs. 84%), the difference was not statistically significant. Thus, VEGF on its own may not have prognostic value in this type of cancer, but may become a useful indicator when combined with other factors, including vascularity and proliferation, which are currently being investigated in our laboratories with a large number of patients.
CONCLUSIONSc-kit is expressed relatively frequently in NSCLC and may become a therapeutic target for patients, particularly those with inoperable or recurrent c-kit positive tumors. The alterations in p53 probably constitute an early event, whereas the abnormalities in c-kit seem to be involved in tumor progression.
References1. Majumder S, Brown K, Qiu FH, Besmer P. c-kit protein, a transmembrane kinase: Identification in tissues and characterization. Mol Cell Biol. 1988;8:4896–4903. PMID: 2463468
2. Ashman LK. The biology of stem-cell factor and its receptor c-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. PMID: 10582338
3. Pietsch T, Kyas U, Steffens U, Ukisan E, Hadam MR, Ludwig WD, Zsebo K, Welte K. Effects of human stem-cell factor (c-kit ligand) on proliferation of myeloid leukemia cells: heterogeneity in response and synergy with other hematopoietic growth factors. Blood. 1992;80:1199–1206. PMID: 1381238
4. Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. PMID: 12094373
5. Lassam N, Bickford S. Loss of c-kit expression in cultured melanoma cells. Oncogene. 1992;7:51–56. PMID: 1371338
6. Strohmeyer T, Peter S, Hartmann M, Munemitsu S, Ackermann R, Ullrich A, Slamon DJ. Expression of the hst-1 and c-kit protooncogenes in human testicular germ cell tumors. Cancer Res. 1991;51:1811–1816. PMID: 1706218
7. Hines SJ, Organ C, Kornstein MJ, Krystal GW. Coexpression of the c-kit and stem cell factor genes in breast carcinomas. Cell Growth Differ. 1995;6:769–779. PMID: 7545433
8. Hibi K, Takahashi T, Nakamura S, Sekido Y, Ueda R, Hida T, Ariyoshi Y, Takagi H, Takahashi T. Coexpression of the stem cell factor and the c-kit genes in small-cell lung cancer. Oncogene. 1991;6:2291–2296. PMID: 1722571
9. Plummer H, Catlett J, Leftwich J, Armstrong B, Carlson P, Huff T, Krystal G. c-myc expression correlates with suppression of c-kit proto-oncogene expression in small cell lung cancer cell lines. Cancer Res. 1993;53:4337–4342. PMID: 7689933
10. Krystal GW, Honsawek S, Kiewlich D, Liang C, Vasile S, Sun L, McMahon G, Lipson KE. Indolinone tyrosine kinase inhibitors block kit activation and growth of small cell lung cancer cells. Cancer Res. 2001;61:3660–3668. PMID: 11325836
11. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. In : Travis W.D., editor. Histological typing of lung and pleural tumors. World Health Organization International Histological Classification of Tumors. Histological Typing of Lung and Pleural Tumors. 1999. 3rd edBerlin: Springer; p. 21–66.
12. Mountain CF. Revisions in the international system for lung cancer. Chest. 1997;111:1710–1717. PMID: 9187198
13. Yoo J, Kang SJ, Ahn WS, Kim BK. E-Cadherin expression and p53 alterations in soft tissue sarcomas: a possible role in epithelial differentiation. Cancer Res Treat. 2001;33:343–349.
14. Joensuu H, Roberts PJ, Sarlomo-Rikala M, Anderson LC, Terrahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitijevic S, Druker B, Demitri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. PMID: 11287975
15. Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res. 2000;6:3319–3326. PMID: 10955819
16. Pietsch T, Nicotra MR, Fraioli R, Wolf HK, Mottolese M, Natali PG. Expression of the c-kit receptors and its ligand SCF in non-small cell lung carcinomas. Int J Cancer. 1998;75:171–175. PMID: 9462703
17. Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. PMID: 1905840
18. Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris CC. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3547–3551. PMID: 7937053
19. Mitsudomi T, Oyama T, Kusano T, Osaki T, Nakanishi R, Shirakusa T. Mutation of the p53 gene as a predictor of poor prognosis in patients in with non-small cell lung cancer. J Natl Cancer Inst. 1993;85:2018–2023. PMID: 8246288
20. Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, Gazdar AF. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–650. PMID: 9989814
21. Tan DF, Li Q, Rammath N, Beak A, Wiseman S, Anderson T, al-Salameh A, Brooks J, Bepler G. Prognostic significance of expression of p53 oncoprotein in primary stage I-IIIa non-small cell lung cancer. Anticancer Res. 2003;23:1665–1672. PMID: 12820438
22. Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, Wu L, Sidransky D. p53 mutations and survival in stage I non-small cell lung cancer: results of a prospective study. J Natl Cancer Inst. 2003;95:926–927. PMID: 12837819
23. Kohno H, Hiroshima K, Toyozaki T, Fujisawa T, Ohwada H. p53 mutation and allelic loss of chromosome 3p, 9p of preneoplastic lesions in patients with non-small cell lung carcinoma. Cancer. 1999;85:341–347. PMID: 10023701
24. Fontanini G, Vignati S, Boldrini L, Chine S, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861–865. PMID: 9815760
25. Baillie R, Carlile J, Pendleton N, Schor AM. Prognostic value of vascularity and vascular endothelial growth factor expression in non-small cell lung cancer. J Clin Pathol. 2001;54:116–120. PMID: 11215279
Fig. 1Immunohistochemical positivity with the anti-c-kit antibody was detected along the cell-surface membrane and/or in the cytoplasm of the squamous cell carcinoma. ABC method (×200). 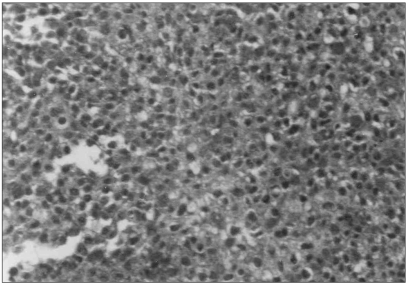
Fig. 2Immunoreactivity with p53 was found in the nucleus of the squamous cell carcinoma. ABC method (×200). 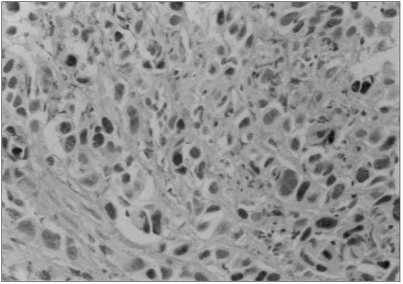
Fig. 3Dense cytoplasmic positivity with VEGF was observed in the large cell undifferentiated carcinoma. ABC method (×200). 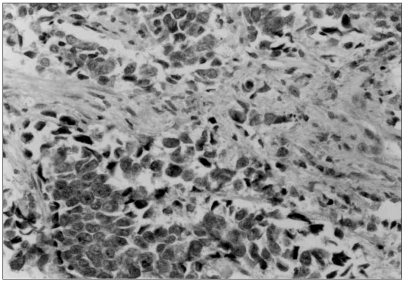
|
|
|||||||||||||||||||||||||||||||||||||||||||