INTRODUCTION
Gastric cancer is one of the most common cancers and the second leading cause of cancer death in Korea (1). A population-based cancer registry was established in January 1, 1997 to estimate the incidence of cancer in Daegu. The age-standardized incidence rates (ASR) of gastric cancer were 69.0/100,000 people for males and 26.9/100,000 people for female by the Daegu Cancer Registry in 1997~1998 (2). Recently, the proportion of gastric cancer cases among all the malignancies is declining; the annual reported cases of gastric cancer was 24.1% of all cancers in 1990, and 20.8% of all cancers in 2000 as reported by the Korea Cancer Registry Program (3), and the decline is principally because of changes in the diet and food preparation, and also the early diagnosis of gastric cancer.
Greater insight has recently been gained into the biological properties of tumor cells. Epidermal growth factor (EGF) promotes the growth of cells from both an ectodermal and mesodermal origin, and EFG plays an important role in cellular proliferation, differentiation and tumor progression of human gastric carcinomas (4). The EGF receptor/ligand system seems to be involved in the regulation of gastric mucosa proliferation and growth regulation of gastric carcinomas (5). Expression of C-erbB-2 genes can easily be detected by immunohistochemical methods in a variety of human malignancies including gastric cancer. (6). C-erbB-2 has been linked to tumor progression. and it is one of the well-known oncogenes involved in the pathogenesis of non-small cell lung cancer. C-erbB-2 amplification and overexpression are currently attracting a great deal of attention because a new adjuvant therapy used against the c-erbB-2 gene product, trastuzumab (Herceptin) has been proved effective in treating the type of breast cancer having an amplification and/or overexpression of c-erbB-2 (7). However, the prognostic role of c-erbB-2 in gastric cancer as an independent marker of poor prognosis still has to be confirmed by further studies.
The purpose of this study was to investigate the prognostic significance of immunohistochemical staining of EGFR and C-erbB-2 in curatively resected gastric adenocarcinoma.
MATERIALS AND METHODS
1) Patients and setting
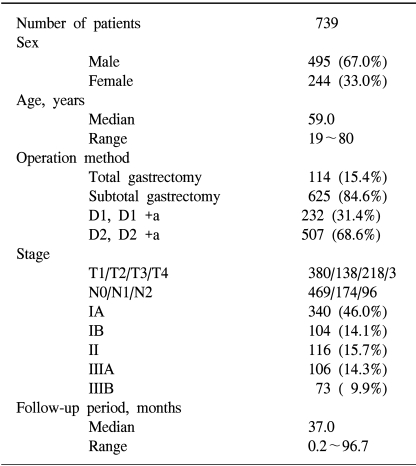
From January 1996 to December 2001, 2,104 pathologically confirmed gastric cancer patients were registered in our hospital. Of these, there were 1,158 curatively resected patients, and immunohistochemical analyses of EGFR and c-erbB-2 were performed in 739 of these patients. We reviewed the clinicopathologic parameters of TNM stage, the World Health Organization classification, histological grade, Lauren classification, Ming classification, vascular invasion and neuronal invasion of these patients. Staging evaluation was done by the guidelines of the American Joint Committee of the Cancer, 5th edition (8).
Curative resection was defined as the removal of all gross tumors and the demonstration of tumor-negative proximal and distal surgical margins by microscopic examination. A total gastrectomy was performed in 114 patients (15.4%), subtotal gastrectomy in 625 patients (84.6%), D1 and D1+α resection was performed in 232 patients (31.4%) and D2 and D 2+α resection was performed in 507 patients (68.6%). Four hundred ninety-five (67.0%) of these patients were male, and 244 (33.0%) of these patients were female. The median age of the subjects was 59.0 years (the range was 19~80 years). The staging was as follows; IA in 340 (46.0%) patients, IB in 104 patients (14.1%), II in 116 patients (15.7%), IIIA in 106 patients (14.3%), and IIIB in 73 patients (9.9%) (Table 1).
2) Immunohistochemical staining
Immunohistochemical staining was performed using the avidin-biotin-peroxidase complex with monoclonal antibodies raised against EGFR (Sigma, diluteed 1:500, St Louis, MO and C-erbB-2 (NCL-L-CB11, diluted 1:40, Novocastra, UK). Representative paraffin blocks containing tumor from each case were sectioned in 5µm slices and affixed to slides and then they were dried for 1 hour at 60℃. The sections were de-paraffined in xylene and rehydrated with a descending series of alcohol concentrations. Endogenous peroxidase activity was blocked by 3% hydrogen peroxidase for 15 min, and this was followed by washing with phosphate buffered saline (PBS), pH 7.2. The sections were then subjected to a heated antigen retrieval process by autoclaving with 1% zinc sulfate solution for 5 min. After cooling for 20 min at room temperature, the sections were incubated with 10% normal horse serum (Vectastain Elite kit) for 30 min. After decanting away the excess serum, the sections were incubated with primary antibody for 2 hour at 37℃. For the EGFR study, Sigma E2760 monoclonal antibody was used at a 1:500 dilution, and for the c-erbB-2 study, the monoclonal antibody NCL-L-CB11 was used at a 1:40 dilution (Novocastra, UK). The sections were subsequently incubated with prediluted biotinylated anti-mouse immunoglobulins (Vectastain Elite kit) for 30 min at 37℃. After washing with PBS, the sections were reacted with peroxidase-conjugated streptoavidin (Dako, Denmark) at a dilution of 1:500 for 30 min at 37℃. After washing with PBS, the peroxidase activity was detected using 3,3'-diaminobenzidine tetrahydrochloride (DAB), and the sections were counterstained with Mayer's hematoxylin.
Two pathologists, who were blind to the clinical outcome and features of the patients, independently evaluated all the immunohistochemical slides. The tumor cells with a positive cytoplasmic staining for EGFR and C-erbB-2 in more than 10% of tumor cells were interpreted as having a positive expression.
3) Statistical analysis
Statistical analysis was performed using χ-2 tests to compare percentages in the cross tabulations, and independent sample T tests were used to compare the means. Survival curves were generated by the Kaplan-Meier method (9), and they were compared by using the log-rank test (10). To determine the significant prognostic factors related to survival and relapse-free survival, multivariate analysis was performed with the Cox proportional hazards regression model (11). All the significance levels refer to two-sided tests. P values less than 0.05 were considered significant.
Statistical analyses were performed using SPSS for Windows 11.0 (Microsoft, Chicago, IL).
RESULTS
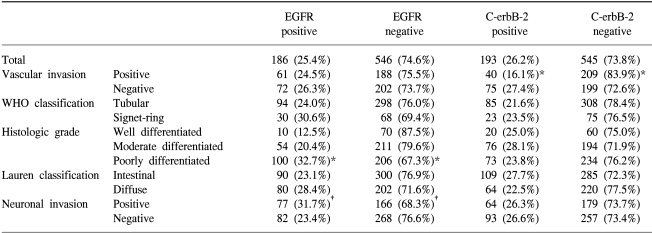
Tumor samples with positive staining for EGFR and C-erbB-2 were found in 186 of the 732 samples (25.4%) and 193 of 738 (26.2%) samples examined, respectively (Table 2). EGFR and C-erbB-2 were expressed in the cytoplasm for all the cases, and C-erbB-2 was more strongly expressed along the cytoplasmic membrane.
1) Correlation with clinicopathologic parameters
According to the World Health Organization classification, overexpression of C-erbB-2 was inversely associated with vascular invasion; vascular invasion was detected in 34.8% of patients with positive c-erbB-2 expressions, and in 51.2% of patients with negative c-erbB-2 expressions (p=0.001). Positive EGFR expressions were associated with more poorly differentiated tumor; there was EGFR expression in 32.7% of poorly differentiated tumor, 20.4% in moderately differentiated tumor and 12.5% in well differentiated tumor, respectively (p=0.000), and in neuronal invasions (p=0.03) (Table 2).
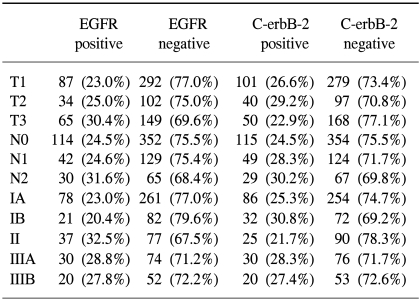
No significant correlation was observed for the overexpression of EGFR and C-erbB-2, and the Lauren classification, Ming classification, depth of invasion (pT category), lymph node involvement (pN category), and pathologic stage (Table 3).
2) Survival analysis
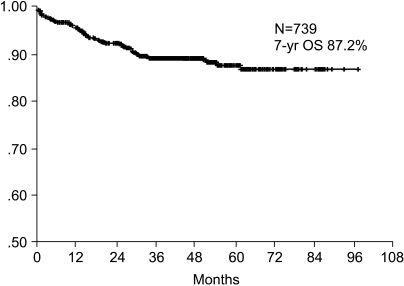
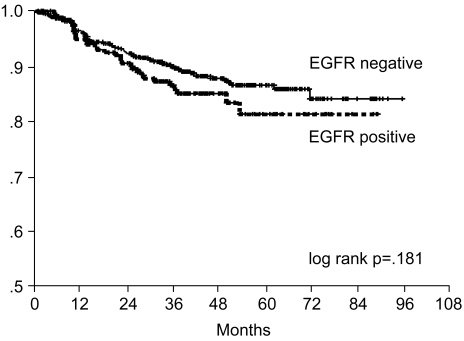
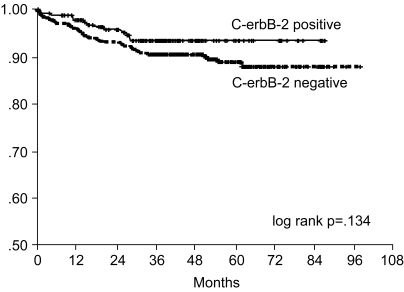
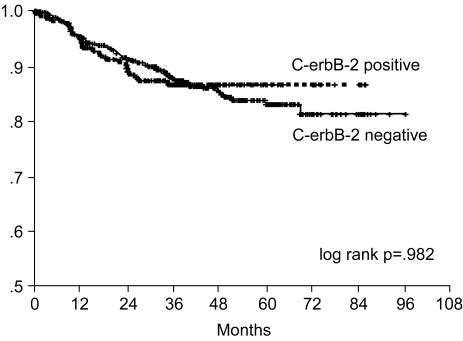
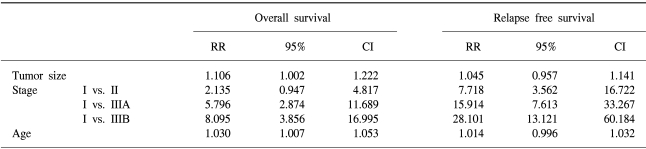
The 7-year overall survival rate (Fig. 1), and the relapse-free survival rate (Fig. 2) were 87.2%, and 79.8%, respectively. There were no significant differences in the overall survival rate and relapse-free survival rates between patients with positive and negative EGFR expression (p=0.954, p=0.181, respectively) (Fig. 3, 4), and positive and negative C-erbB-2 expression (p=0.134, p=0.982, respectively) (Fig. 5, 6). According to multivariate Cox regression analysis, the tumor stage, size and age were important prognostic factors for overall survival, and tumor stage was important for the relapse-free survival. Positive expression of EGFR and C-erbB-2 were not independent prognostic factors for survival rates (Table 4).
DISCUSSION
Among the various malignancies, gastric cancer is the second most common cause of cancer death through out the world, and the prognosis for advanced gastric cancer is still very poor. The human C-erbB-2 gene is an oncogene encoding a 185-kDa glycoprotein that belongs to the transmembrane type 1 tyrosine kinase receptor family that includes the EGF receptors, HER3 and HER4. The increased expression of EGF receptor protein in gastric cancer appears to be related to the tumor's biologic aggressiveness (12). C-erbB-2 is frequently overexpressed in the adenocarcinomas of breast, ovary, lung and stomach (6). Although the mechanism of erbB-2 overexpression is thought to be the result of transcriptional upregulation in many primary human carcinomas, the expression rate of c-erbB-2 or its mRNA level is usually lower than the level of the translated protein (13). Immunohistochemical membrane staining for c-erbB-2 protein has been reported to correspond well to gene amplification and the expression of protein (14), and in the current study, immunohistochemical staining of c-erbB-2 protein was usually found in the cytoplasm; stronger staining on the plasma membrane of cells in the intestinal metaplasia was found and membrane staining was detected only in the cancer cells.
In the pervious studies, the positive rate for c-erbB-2 expression in gastric cancer was 12-34% (15~17) and the c-erbB-2 expression rate for gastric cancer was 26.2% in our study. In regards to the WHO histopathologic type, the rate for positive immunohistochemical staining of the c-erbB-2 protein was significantly higher for the tubular cell type gastric cancer than signet ring cell type of gastric cancer (18,19). No correlation was demonstrated in our study between the expression of c-erbB-2 and the WHO histopathologic types of gastric cancer. The incidence of c-erbB-2 expression is higher for the intestinal type than for the diffuse carcinoma (39.7% vs. 18.4%) (15), but our study did not show any difference between the Lauren classification and the C-erbB-2 expressions. For well-differentiated adenocarcinoma, the incidence of c-erbB-2 expression was shown to be higher than for the poorly differentiated carcinomas (20), but in our study, the expression of c-erbB-2 and the histologic tumor grade was not related. Vascular invasion had an inverse correlation to c-erbB-2 expression in our study; vascular invasion was detected for 34.8% of patients with positive c-erbB-2 expression and in 51.2% of patients with negative c-erbB-2 expressions (p=0.001).
In the previous studies, EGFR expression was positive for 38% of the gastric tumors (21), and in our study, there was 25.4% rate of expression in the gastric tumors. Positive EGFR expressions were strongly associated with poorly differentiated tumors, and this is a similar result as was found in Koullias's study (21); there was 32.7% EGFR expression in the poorly differentiated tumor, 20.4% EGFR expression in moderately differentiated tumor and 12.5% EGFR expression in well differentiated tumor, respectively (p=0.000). The positive EGFR expression in gastric cancer was correlated with the histologic grade (p=0.000) and the neuronal invasion status (p=0.030).
There was no significant correlation found between age, sex, tumor size, depth of tumor invasion, lymph node metastasis and overexpressions of c-erbB-2 and EGFR in our study of curatively resected gastric cancer patients.
In our current study, the 7-year survival rate and relapse-free survival rate were 87.2% and 79.8% respectively. It has been found that patients with c-erbB-2 positive tumors had poor survival rates in many studies (12,15,20,22), and the relative risk of death for patients with c-erbB-2-positive tumors was increased 5-fold (20). The c-erbB-2 marker is a useful indicator of poor prognosis for the patients with well-differentiated adenocarcinomas (20) but not for patients with poorly differentiated carcinomas, and patients with c-erbB-2-positive tumors had poor survival rates in the early stages (23), but not in the advanced stages. However, in several Korean studies, no significant association was found between c-erbB-2 status and survival duration (24,25), which is the same result as found in our study. The survival rate was significantly lower for patients with EGFR positive tumors in several studies (12), but there was no significant relationship between the EGFR levels of the tumors and the overall survival in other studies (4), as was also the case in our study.
By the multivariate Cox regression analysis, stage, tumor size, and age were the important prognostic factors for overall survival, and tumor stage was the important prognostic factor for relapse-free survival. Currently, the most accurate prognostic indicator for gastric cancer is the tumor stage. In our study, there was no significant difference on the subset analysis according to Lauren histological type.