AbstractPurposeCaspase-3 is a cysteine protease that plays an important role in the process of apoptotic cell death, but little has been studied clinically on caspase-3 in lung cancer. Increased c-myc expression can result in mitosis or apoptosis, and its contribution to the pathogenesis and prognosis of lung cancer has gained interest. In the present study, the expressions of caspase-3 and c-myc, along with their possible correlations with prognostic variables, were analyzed in resected non-small cell lung carcinomas (NSCLC).
Materials and MethodsArchival tumor tissues from 147 previously untreated NSCLC patients were examined by immunohistochemistry for the expressions of caspase-3 and c-myc proteins. Clinical information was obtained through the computerized retrospective database from the tumor registry.
ResultsThe expressions of caspase-3 and c-myc were detected in 60 (88/147) and 16% (24/147) of tumors, respectively. No association was found between caspase-3 and c-myc expressions. A multivariate analysis demonstrated the N status and pathologic stage to be significantly correlated with poor survival (p-value=.018 and .002, respectively), but positive expression of caspase-3 was associated with a good prognosis (p=.03).
INTRODUCTIONIn cancer, two major factors determine the kinetics of cell turnover: cellular proliferation and apoptosis (programmed cell death). Dysregulation of their cellular signaling pathways can result in cellular transformation and cancer. Dysregulation of apoptotic cell death has evolved as a critical key player, not only for tumorigenesis, but also in the biological behavior of cancer (1). The present study focuses on two molecules playing important roles in the cellular pathways affecting apoptosis.
Recent studies have implicated a family of cysteine proteases, called caspases, as being associated with the induction of apoptosis; the activated caspases cleave key substrates to induce apoptotic cell death. The activation of at least one caspase appears to be an essential step in cellular apoptosis. Caspase-3 (also known as CPP32, YAMA and apopain) is synthesized as an inactive proenzyme, which upon activation, is cleaved at the ASP28-Ser29 and ASP175-Ser176, generating two subunits of 17kDa and 12kDa, respectively. The activated caspase-3 abrogates the effect of substrates that protect cellular integrity, such as the DNA-repair enzyme poly (ADP-ribose) polymerase (PARP), thereby inducing apoptotic cell death (2). Caspase-3 has been reported to be expressed in some malignant tumors, including leukemia, malignant lymphoma, neuroblastoma and glioma (3~6).
Increased c-myc expression can result in mitosis or apoptosis, depending on the availability of other critical growth stimuli. In the presence of such stimuli, c-myc acts as a classic protooncogene, stimulating mitosis; in their absence, it initiates apoptosis (7). Because the dysregulated expression of c-myc contributes to the tumorigenesis of cervix, breast and colon cancers, there has been growing interest in analyzing its contribution to the pathogenesis and prognosis of lung cancer (8~10).
Lung cancer is a major cause of cancer death worldwide, and has become the leading cause of cancer death in Korea. Although recent molecular studies have provided increased understanding of the biology of lung cancer, the essential genetic features, along with factors for prognosis, remain to be determined. To date, the literature contains limited data regarding caspase-3 and c-myc in lung cancer. In this study, the expressions of those proteins in formalin-fixed, paraffin-embedded, lung tumor tissues from patients with non-small cell lung carcinoma (NSCLC) were analyzed by immunohistochemistry. The resulting data were evaluated for possible correlations between their expressions and clinicopathological parameters.
MATERIALS AND METHODSThe tumor tissue samples were obtained from 147 NSCLC patients who underwent surgical resection at the Catholic University St Vincent's Hospital between January 2001 and December 2002. No pre-operative radio- or chemotherapy had been performed. Clinical information was obtained through a computerized retrospective database of the tumor registry. All patients had been followed up for overall survival. Follow-up data were available for a time period ranging from 4 to 169 weeks, with a mean of 92 weeks. Thirty-six patients died during follow-up, but 111 were alive at the time of the study.
Tissue samples were fixed in 10% buffered formalin. After routine embedding, light microscopy led to the final diagnosis. One of the authors (JY) reviewed the histopathological diagnosis, according to the relevant WHO classifications, tumor grade and quality of the tissue sections. For immunohistochemical staining, a sensitive peroxidase-streptavidin method, as described previously (11), was performed using the representative sections of archival, formalin-fixed, paraffin-embedded tumor specimens. Briefly, each block was cut into 4 µm thick sections, which were deparaffinized in xylene and rehydrated in graded alcohols and water. Endogenous peroxidase was blocked by soaking in 3% H2O2 at 45℃ for 4 min. The slides were heated to 120℃ in a citrate buffer (2.1g/L, pH 6.0) for 15 min to unmask the antigen, and then treated with a protein blocking reagent before overnight incubation, with primary antibodies at a 1:50 dilution, at 4℃, as recommended by the supplier. A polyclonal anti-caspase-3 antibody was purchased for the expression of caspase-3 (Pharmingen, San Diego, CA). This antibody recognizes both the inactive 32 kDa caspase-3 and the active 17 kDa fragment. Another primary antibody used in the present study was that of anti-c-myc (NeoMarkers, Fremont, CA). After extensive washing, the sections were incubated at room temperature for 10 min, with biotinylated anti-mouse immunoglobulin antibodies (Zymed, San Francisco, CA) at a 1:20 dilution, and subsequently with streptavidinbiotin peroxidase complexes at a 1:25 dilution. The reaction products were visualized by immersing slides in 3, 3'-diaminobenzidine tetrahydrochloride and finally counterstained with hematoxylin.
All series included positive and negative controls. The tonsil and tissue from breast carcinomas were used as the positive controls for caspase-3 and c-myc, respectively. Negative controls of the immunohistochemistry technique were performed by replacing the primary antibodies with dilution buffer. The immunoreactivity for caspase-3 was considered positive if predominantly cytoplasmic, with some nuclear staining. Tumor cells showing a nuclear staining pattern were interpreted as positive for c-myc. Three observers initially reached agreement in 89% of cases. For the discordant samples, slides were reviewed jointly, and a consensus reached.
Statistical analysis was carried out using the SSPS 11.5 software package (Seoul, Korea). Survival was measured in weeks from the date of surgery. The influence of various clinicopathological factors, including caspase-3 and c-myc expression indices, on the survival was assessed with a Cox proportional hazards model. Two-sided P values were determined by means of the log-rank test. The level of significance was set at 0.05.
RESULTSThe clinical characteristics, along with the results of immunohistochemical staining, are listed in Table 1. There were 106 men and 41 women, with a mean age of 66 years, ranging from 33 to 87. The T status distribution was as follows: 8% T1, 33% T2, 8% T3 and 51% T4. Twenty-two patients had no lymph node involvement. All the patients were staged at the time of their surgery, according to the guidelines of the American Joint Committee on Cancer. There were 15 patients in stage I, 5 in stage II and 127 in stage III. According to the histologic type, 61 were adenocarcinomas, 63 squamous cell carcinomas and 23 large cell carcinomas.
Negative and positive controls for immunohistochemistry showed the expected results. Caspase-3 expression was identified in 88 patients (60%) (Fig. 1A), whereas c-myc was observed in 24 (16%) (Fig. 1B). No correlation was found between caspase-3 expression and c-myc activation.
The median survival time was significantly longer in patients with positive caspase-3 expression than in the negative patients (66 vs. 27 weeks). In contrast, the patients with c-myc positive tumors had a median survival time of 28 weeks; while this was 34 weeks in c-myc negative patients (data not shown), but the difference was not statistically significant. A multivariate analysis of the clinical and immunohistochemical data is summarized in Table 2. Positive expression of caspase-3 was significantly correlated with a good prognosis (p=.03), whereas c-myc showed no association with survival (Fig. 2). It was indicated that the N status, pathologic stage and caspase-3 expression were independent prognostic factors.
To determine whether the combination of caspase-3 and c-myc expressions had any additional prognostic value, patients were grouped with respect to their expression stati of both variables. The median survival time for patients with caspase-3 positive/c-myc-positive, caspase-3-positive/c-myc-negative, caspase-3-negative/c-myc-positive and caspase-3-negative/c-myc-negative tumors were 47, 59, 28 and 38 weeks (data not shown), respectively. This difference, however, was not statistically significant.
DISCUSSIONMultiple factors are responsible for the modulation of tumor growth and the prognosis of patients with malignant tumors. Cellular proliferation and apoptosis are two major factors determining the kinetics of cell turnover in cancer. An imbalance between these factors is believed to underlie tumor development and prognosis. Caspase-3 is one of the most important molecules in the apoptosis cascade (12), but the association between caspase-3 expression and prognosis of various malignancy types is controversial. In esophageal squamous cell carcinomas, caspase-3 expression was present in 60% of tumors, and correlated with a favorable prognosis (13). High levels of caspase-3 mRNA expression in neuroblastomas were also associated with a favorable prognosis (5). In contrast, a significant correlation with a higher risk of recurrence, as well as no association with prognosis, was reported in colorectal and liver cell carcinomas, respectively (14,15).
In the present study, caspase-3 was frequently expressed in NSCLC, suggesting its possible involvement in tumor development. In addition, caspase-3 positive staining was a significant prognostic factor in predicting survival in these tumors; the median survival was longer for caspase-3 positive than for caspase-3 negative patients (66 vs. 27 weeks, p=.019). This was in keeping with the results of Koomagi et al. (16,17), who found caspase-3 expression in 72% of NSCLC, with a favorable prognosis. Takata et al.(18) demonstrated 58% (69/118) caspase-3 immunoreactivity in lung cancer, which was consistent with the results of our study (60%). However, in their study, the 5-year survival rate for caspase-3 positive patients was significantly lower than that for caspase-3 negative patients (66.6 vs. 82.1%, p=.049). The reason for a poor prognosis in patients with caspase-3 expression was explained as a result of decreased caspase-3 mediated apoptotic cancer cell death; since the antibodies they used recognized the "uncleaved" inactive form only, with most of the caspase-3 expression considered as the inactive form. Caspases are synthesized as "uncleaved" proenzymes. Cleavage at specific aspartate residues converts the proenzymes into biologically active cysteine proteases. Therefore, apoptotic cell death mediated by caspase-3 is induced only after the inactive 32 kDa caspase-3 is cleaved into two active fragments of 17 and 12 kDa, respectively. However, the antibodies used in the study of caspase-3 expression with a favorable prognosis also react, not with the "cleaved" caspase-3, but with the "uncleaved" caspase-3 (16). Furthermore, the polyclonal anti-caspase-3 antibodies used in our study recognized both the pro- and activated caspase-3. The discrepancies in the prognostic significance of caspase-3 activity, thus, may not be due to the use of different primary antibodies. Further studies on the expression of the "uncleaved" and "cleaved" caspase-3 in a larger series, with a long-term follow-up, will clarify the role of the caspase-3 protein in the prognostic implications of lung cancer.
The c-myc oncogene is known to regulate neoplastic development and apoptotic cell death (7). Deregulation of c-myc occurs in a broad range of human tumors, including lung cancer. C-myc not only promotes G1 to S cell cycle progression in a mechanism involving activation of cyclin-E/cdk2, but also sensitizes cells to apoptosis. Its expression in the determination of prognostic significance has infrequently been investigated in NSCLC. Amplification of c-myc was reported to be associated with lymph node metastasis, but not with the clinical outcome (19,20). It was, however, recently shown that c-myc amplification was related to a shortening of survival (21). Our study demonstrated no difference in the median survival time of patients with and without c-myc expression (28 weeks vs. 34 weeks), suggesting that c-myc alone may not contribute critically to the progression of NSCLC. Other gene products and/or additional factors dictating the outcome of c-myc expression on survival may be required.
Interestingly, our patients with caspase-3-positive and c-myc-negative tumors had a tendency for the most favorable prognosis, while patients with caspase-3-negative and c-myc-positive tumors tended to have the most unfavorable prognosis. Although the combination of both gene expression stati provided no statistically significant prognostic information, extended analyses, including these proteins and other associated gene products, may aid in the understanding of the novel mechanism for complex molecular control in lung cancer, which may yield useful prognostic information.
NotesThis work was supported in part by the 2004 Research Fund from the St. Vincent's Hospital and from the Lung Cancer Study Group, St. Vincent's Hospital. References1. Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. PMID: 8156506
2. Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (Caspase-3), a cell death protease. Cancer Res. 1997;57:1605–1613. PMID: 9108467
3. Estrov Z, Thall PF, Talpaz M, Estey EH, Kantarjian HM, Andreeff M, Harris D, Van Q, Walterscheid M, Kornblau SM. Caspase 2 and caspase 3 protein levels as predictors of survival in acute myelogenous leukemia. Blood. 1998;92:3090–3097. PMID: 9787143
4. Donoghue S, Baden HS, Lauder I, Sobolewski S, Pringle JH. Immunohistochemical localization of caspase-3 correlates with clinical outcome in B-cell diffuse large-cell lymphoma. Cancer Res. 1999;59:5386–5391. PMID: 10537324
5. Nakagawara A, Nakamura Y, Ikeda H, Hiwasa T, Kuida K, Su MS, Zhao H, Cnaan A, Sakiyama S. High levels of expression and nuclear localization of interleukin-1beta converting enzyme (ICE) and CPP32 in favorable human neuroblastomas. Cancer Res. 1997;57:4578–4584. PMID: 9377572
6. Kondo S, Tanaka Y, Kondo Y, Ishizaka Y, Hitomi M, Haqqi T, Liu J, Barnett GH, Alnemri ES, Barna BP. Retroviral transfer of CPP32beta gene into malignant gliomas in vitro and in vivo. Cancer Res. 1998;58:962–967. PMID: 9500457
7. Wyllie AH. Apoptosis (The 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993;67:205–208. PMID: 8431353
8. Barr LF, Campbell SE, Diette GB, Gabrielson EW, Kim S, Shim H, Dang CV. c-Myc suppresses the tumorigenicity of lung cancer cells and down-regulates vascular endothelial growth factor expression. Cancer Res. 2000;60:143–149. PMID: 10646866
9. Prins J, De Vries EG, Mulder NH. The myc family of oncogenes and their presence and importance in small-cell lung carcinoma and other tumour types. Anticancer Res. 1993;13:1373–1385. PMID: 8239508
10. Gosney JR, Field JK, Gosney MA, Lye MD, Spandidos DA, Butt SA. c-myc oncoprotein in bronchial carcinoma: expression in all major morphological types. Anticancer Res. 1990;10:623–628. PMID: 2164348
11. Yoo J, Kang SJ, Ahn WS, Kim BK. E-Cadherin expression and p53 alterations in soft tissue sarcomas: a possible role in epithelial differentiation. Cancer Res Treat. 2001;33:343–349.
12. Depraetere V, Golstein P. Dismantling in cell death: molecular mechanisms and relationship to caspase activation. Scand J Immunol. 1998;47:523–531. PMID: 9652819
13. Hsia JY, Chen CY, Chen JT, Hsu CP, Shai SE, Yang SS, Chuang CY, Wang PY, Miaw J. Prognostic significance of caspase-3 expression in primary resected esophageal squamous cell carcinoma. Eur J Surg Oncol. 2003;29:44–48. PMID: 12559076
14. Jonges LE, Nagelkerke JF, Ensink NG, van der Velde EA, Tollenaar RA, Fleuren GJ, van de Velde CJ, Morreau H, Kuppen PJ. Caspase-3 activity as a prognostic factor in colorectal carcinoma. Lab Invest. 2001;81:681–688. PMID: 11351040
15. Persad R, Liu C, Wu TT, Houlihan PS, Hamilton SR, Diehl AM, Rashid A. Overexpression of caspase-3 in hepatocellular carcinomas. Mod Pathol. 2004;17:861–867. PMID: 15098015
16. Koomagi R, Volm M. Relationship between the expression of caspase-3 and the clinical outcome of patients with non-small cell lung cancer. Anticancer Res. 2000;20:493–496. PMID: 10769711
17. Volm M, Koomagi R. Relevance of proliferative and pro-apoptotic factors in non-small-cell lung cancer for patient survival. Br J Cancer. 2000;82:1747–1754. PMID: 10817513
18. Takata T, Tanaka F, Yamada T, Yanagihara K, Otake Y, Kawano Y, Nakagawa T, Miyahara R, Oyanagi H, Inui K, Wada H. Clinical significance of caspase-3 expression in pathologic-stage I, non small-cell lung cancer. Int J Cancer. 2001;96(suppl):54–60. PMID: 11992386
19. Kubokura H, Tenjin T, Akiyama H, Koizumi K, Nishimura H, Yamamoto M, Tanaka S. Relations of the c-myc gene and chromosome 8 in non-small cell lung cancer: analysis by fluorescence in situ hybridization. Ann Thorac Cardiovasc Surg. 2001;7:197–203. PMID: 11578259
20. Volm M, Koomagi R, Mattern J, Efferth T. Protein expression profile of primary human squamous cell lung carcinomas indicative of the incidence of metastases. Clin Exp Metastasis. 2002;19:385–390. PMID: 12198766
21. Yakut T, Egeli U, Gebitekin C. Investigation of c-myc and p53 gene alterations in the tumor and surgical borderline tissues of NSCLC and effects on clinicopathologic behavior: by the FISH technique. Lung. 2003;181:245–258. PMID: 14705768
Fig. 1Immunohistochemical staining of caspase-3 (A) and c-myc (B) in primary resected NSCLC. The majority of tumor cells show immunoreactivity in their cytoplasm with some nuclear staining (A), and intense nuclear immunostaining (B) (magnification × 100). 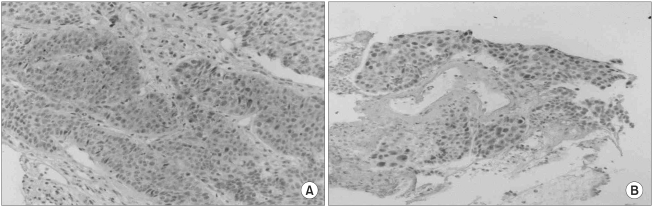
|
|
||||||||||||||||||||||||||||||||||||