INTRODUCTION
After the transurethral resection of superficial bladder cancer, the recurrence rate is 40% to 80% and the progression rate to muscle invasion or distant metastasis is 5% to 25% (1). Intravesical bacillus Calmette Guerin (BCG) instillation remains the standard treatment among intravesical anticancer therapies after transurethral resection for bladder cancer, to suppress disease recurrence and progression (2). However, the toxicity of BCG is a limitation in some patients. Thus, alternative intravesical chemotherapeutic agents have been developed for patients who develop toxic symptoms or for those with a relatively lower risk of recurrence or progression.
Mitomycin C (MMC) is one such agent. However, because there is no standard treatment duration or dose, various doses and schedules have been adopted by researchers. In particularly, no study has compared dosage efficacy and side effects in Koreans. Nevertheless, in Korea, 40 mg of MMC was de facto standard as yet. Therefore, we believed that the optimal dose determination of intravesical MMC instillation in Koreans among 30 mg and 40 mg, widely adopted in the other countries, would provide fundamental information for the prophylactic treatment of superficial bladder cancer in Korea. We performed this prospective, randomized study to compare prophylactic efficacy and safety of intravesical MMC instillation at two different doses (30 mg or 40 mg) after transurethral resection for bladder cancer in Korean superficial bladder cancer patients.
MATERIALS AND METHODS
Enrollment commenced in September 1998 and continued through December 2002. Patients, 75 years old or younger, diagnosed by pathology as having superficial transitional cell carcinoma (Tis, Ta, and T1, any grade) after complete transurethral resection of bladder tumor in our institution were included in the study. It must be initial case or recurred case who failed intravesical anticancer therapy except MMC. The exclusion criteria included prior surgical management more than 3 occasions, other prior treatments except surgical management (i.e., chemotherapy, immunotherapy, and radiation therapy), severe cardiac failure, renal failure, liver failure, hematopoietic disease, severe complication after transurethral resection of bladder tumor, and concomitant cancer. A total of 97 patients were finally included.
To determine the optimal MMC instillation dose, patients were randomly divided into two groups by telephone number. One group (n=53) received 30 mg of MMC, and the other group (n=44) received 40 mg of MMC. We mixed 30 mg of MMC with 30 ml of normal saline for the first group, and 40 mg of MMC with 40 ml for the second group. The treatment schedule was once per week for first 8 weeks, and once per month for the following 10 months as a maintenance therapy, a total of 18 instillations. After intravesical MMC instillation, we changed a patient's position, and 2 hour later allowed patients to void. A cystoscopic examination, complete blood count, serum chemistry including liver function test, urinalysis, urine cytology, and checking side effect were performed every 3 months after MMC treatment commencement. The patients supplied written informed consent to a document describing the investigational nature of the protocol.
We defined recurrence as the occurrence of a pathologic proven new superficial bladder carcinoma, or transitional cell carcinoma in situ by biopsy or transurethral resection of bladder tumor during periodic cystoscopic examination. Progression was defined as more than bladder muscle invasion or distant metastasis.
We assumed that recurrence rate after 40 mg MMC instillation is 30% as previous our institution experience, to obtain 80% power of obtaining a significant result (p<0.05) with 25% difference interval, the required sample size was 42 persons per group.
We used Chi-square test, the linear-by-linear test, and the Student's t-test for statistical analyses. Survival rates were studied using the Kaplan-Meier method and comparisons were made using the log-rank test. Resulting p values are reported and significance was assumed at the 0.05 level.
RESULTS
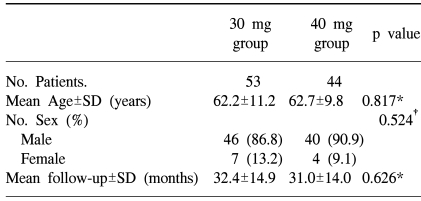
There were no significant differences between the two groups, in terms of sex, age, and mean duration of follow-up (Table 1).
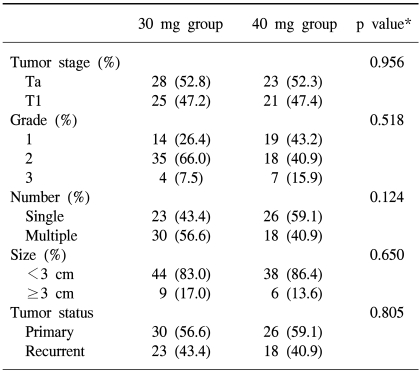
In the 30 mg group there were 28 Ta (52.8%), and 25 T1 (47.2%) stage, and in the 40 mg group there were 23 Ta (52.3%), and 21 T1 (47.7%) stage. In the 30 mg group there were 14 grade I (26.4%), 35 grade II (66.0%), and 4 grade III (7.5%), and in the 40 mg group there were 19 grade I (43.2%), 18 grade II (40.9%), and 7 grade III (15.9%). There were no significances between the two groups in stage (p=0.956) or grade (p=0.518)(Table 2).
During follow-up, the overall one and two year recurrence rates were 19% and 24% in the 30 mg group, and 12% and 22% in the 40 mg group, which was not significantly different. The total recurrence rate was 32% (31 of 97 patients), in the 30 mg group it was 37.7% (20 of 53 patients), and in the 40 mg group it was 25.0% (11 of 44 patients). It appeared like that the 30 mg group had a higher recurrence rate than the 40 mg group, but this was not significant. Median time to recurrence was 13.5 months (range: 3~44) in the 30 mg group, and 12.0 months (range: 5~29) in the 40 mg group, which was not statistically different. No significant difference was observed between the two groups in terms of the recurrence-free rate (p=0.171)(Fig. 1).
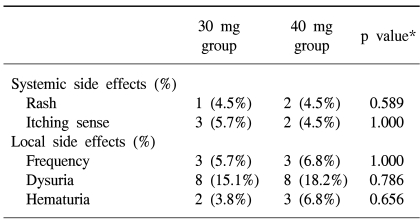
Side effects were reported in 26 of the 97 patients (26.8%). The toxicity rates were 22.6% in the 30 mg group and 31.8% in the 40 mg group, however there was no statistical significance (Table 3). Treatment was not discontinued or postponed in any subject, and all side effects disappeared spontaneously without treatment. During the treatment periods, no abnormal complete blood count, serum chemistry or liver function test results were observed.
DISCUSSION
In cases of superficial bladder cancer, after transurethral resection of bladder tumor, 50~70% recur (3) and 4~10% progress to invasive cancer (4). Studies of intravesical instillation after transurethral resection for superficial bladder tumor have been performed with a variety of chemotherapeutic agents and immunologic agents, such as MMC, doxorubicin, epirubicin, thiotepa, valruvicin, ethoglucid and BCG, to lower the recurrence rate (5). Of these drugs, MMC was first reported in 1975, and a 70~90% suppressive effect was reported on superficial bladder cancer recurrence (6). Thereafter one study reported that MMC instillation significantly decreased recurrence at the short-term follow-up (7). MMC is an alkylating agent with a molecular weight of 334 kd. Its mechanism of action has not been totally clarified, although some evidence suggests that it acts by binding to DNA, resulting in synthesis inhibition and strand breakage. MMC is most sensitive in the late G1 and early S phase, but overall it is considered cell cycle-nonspecific (8). However, because no consensus has been reached on the optimal dose of MMC instillation, a variety of doses from 20mg to 80mg have been employed and no standard treatment schedule has been established. 30 mg and 40 mg of MMC were mostly used dose internationally (9). In Korea, 40 mg of MMC was de facto standard as yet (10~12). But there has been no study compared dosage efficacy and side effects in Koreans.
In this study, we used 30 mg or 40 mg of MMC, and a weekly treatment schedule for the first 8 weeks and a monthly schedule for the following 10 months as a maintenance therapy. According to our results, after intravesical MMC instillation treatment the recurrence rate was 25% for both groups, which suggests that the prophylactic effects are same. However, one study compared the recurrence rates of superficial bladder cancer for 30 mg of thiotepa mixed in 30 ml normal saline group and 40 mg of thiotepa mixed in 40 ml of normal saline, and reported that the effect of thiotepa on recurrence was determined by not total dose but by its concentration (13). On the other hand, other researchers performed a study in which they administered different concentrations (1 mg/ml or 2 mg/ml) of epirubicin to superficial bladder cancer patients intravesically, and they reported that the effect of epirubicin was dose-dependent, because they observe similar response rates (14).
MMC is one of antibiotic agents which produce alkylating agent by intracellular action. Because its molecular weight is large, 334Da, its absorption into the bladder is less than BCG, and it is known that its systemic side effects are less frequent than those of BCG. As similar as previously reported study, after 40 mg of MMC intravesical instillation, 5.2% of patients showed skin eruption and 1.7% experienced an itching sense as systemic symptom, 28% showed bladder irritative symptoms, 33% hematuria, and 8.7% experienced painful voiding as local symptoms (15). Skin response due to a delayed hyperreactive response to MMC was observed in 10% of patients (16). In the present study, we observed skin eruption in 4.1%, an itching sense in 4.1%, bladder irritative symptoms in 6.1%, hematuria in 5.1%, and painful voiding in 16.3%, which agree with the results of previous study (15). Moreover, no significant difference was observed between the side effects experienced by the 30 mg group and the 40 mg group. Treatment was not discontinued or postponed for any patient due to side effects, and every side effect disappeared spontaneously without treatment.
CONCLUSIONS
In Korean superficial bladder cancer patients, the prophylactic effect and the side effect rates of MMC instillation were similar at doses of 30 mg and 40 mg. Our results show that one could consider 30 mg instead of 40 mg as an intravesical MMC instillation dose to decrease recurrence in Korean superficial bladder cancer patients. However, because this study was underpowered statistically, we think a larger scale study is required.