AbstractPurposeThis study was carried out to assess the usage of an in vivo hollow fiber assay to screen drugs with highly predictive accuracy.
Materials and MethodsThe assay systems used were the hollow fiber and xenografts assays. The hollow fiber assay was carried out with the following steps; preparation of fibers, preparation of cells, loading and implanting fibers, treatment with drugs, removal of fibers and assaying for the cell viability by the MTT assay. For the xenografts assay, cell suspensions were subcutaneously transplanted into the mice. Therapy was started when the tumor volume reached 100~200 mm3. The tumor volumes were calculated using the formula V=[length+(width)2]/2, and used for evaluating the efficacy of the drugs. The drug treatment doses used were adriamycin 2.1 mg/kg, mitomycin-C 0.25 mg/kg, 5-fluorouracil 24.5 mg/kg and paclitaxel 2.5 mg/kg, and administrated intravenously five times daily.
INTRODUCTIONThe xenografts model, although actively used in developing chemotherapeutic agents, is costly, time consuming and with poor performance for the transimplantation of human tumor cell lines. To streamline this process, Hollingshead et al. developed a method for drug screening based on implanting tumor cells cultured in polyvinylidine fluoride (PVDF) hollow fibers into athymic mice (1). The hollow fiber assay is a unique in vivo model, which allows simultaneous evaluation of up to 6 different cell lines in 2 physiological separate compartments. The hollow fiber model has a shorter evaluation time and reduced compound requirement than traditional xenografts models. The model allows for the effective pairing of a novel compound, with the appropriate cell line, by its capacity to utilize multiple cell lines.
The hollow fiber assay is used by the National Cancer Institute (NCI) of the National Institute of Health (NIH) for testing compounds as anti-cancer agents. It is also being employed in looking for antiviral compounds for HIV. One advantage of this assay is its unique model of permitting the simultaneous evaluation of compounds against various cell lines at subcutaneous and intra-peritoneal sites (2).
We tested the anti-cancer agents using both the hollow fiber and xenografts assays against various human tumor cell lines in order to evaluate any correlation with regard to the feasibility of the hollow fiber assay for anti-cancer drug screening.
MATERIALS AND METHODS1) Hollow fiberPolyvinylidene fluoride (PVDF) hollow fibers, with a 500,000 Da molecular weight cut-off and 1.0 mm ID (Spectrum Laboratories, Inc, USA), were used in these studies (1). The fibers were flushed under sterile conditions and incubated in RPMI 1,640 medium, containing 10% FBS, for a minimum of 12 hr at 37℃, prior to loading with cells.
2) Cell linesThe human tumor cell lines used in this study were as follows; melanoma SK-MEL-2, cervix adenocarcinoma HeLa and carcinoma SiHa, breast adenocarcinoma MCF-7 and MDA-MB-231, ovary adenocarcinoma SK-OV-3, pancreas adenocarcinoma Capan-1 and Capan-2, colon adenocarcinoma Colo-320DM, WiDr and carcinoma HCT116, lung carcinoma A549 and H209, larynx carcinoma A431, stomach carcinoma MKN-28 and MKN-45, prostate carcinoma PC-3, cervical carcinoma KB, pharynx carcinoma FaDu and liver hepatoblastoma Hep-G2. All cell lines were adapted to a standard culture medium. The cell lines, HeLa, MCF-7, Hep-G2, KB, Colo-320DM, WiDr, HCT116 and SK-MEL-2, were maintained in a minimum essential medium (MEM), supplemented with 10% foetal bovine serum (FBS, JRH Bioscience). The SK-OV-3 cell line was maintained in McCoy's 5a medium, supplemented with 10%
FBS.
3) Animals5 weeks old BALB/C nu/nu mice (male), purchased from Japan SLC, Inc., were acclimatized under the controlled standard condition (temperature, 23±2℃; relative humidity, 50±5%; illumination cycle, 12 h/12 h light/dark), and housed in polycarbonate cages for a week prior to the experiment. Mice were maintained according to accredited procedures in our facility, and fed irradiated Samyang (Korea) chow and UV sterilized water ad libitum.
4) Drug treatmentAll drugs were purchased from Sigma Chemical Company. Chremophor EL, for the preparation of the paclitaxel injection, was also from Sigma Chemical Company. The treatment doses used were adriamycin 2.1 mg/kg, mitomycin-C 0.25 mg/kg, 5-luorouracil 24.5 mg/kg and paclitaxel 2.5 mg/kg, and administrated intravenously five times daily. The dose selection was based on 1/10 of the evaluated LD50.
5) Hollow fiber assayFor the assay, capsules were prepared as follows (3,4): Prior to filling with cells, each fiber was individually rinsed with ice-cold fresh RPMI 1,640, containing 20% FBS. The cell suspension was drawn into a 5 ml syringe, and the fibers filled with the cell suspension via a 20-gauge needle. After filling, the ends of the fibers were heat-sealed, with individual fibers filled with cells prepared by heat-sealing the fibers at 2 cm intervals. Heat sealing was accomplished by clamping the fibers with hot smooth-jawed needle holders. Prior to implantation of capsules into the mice, the capsules were incubated overnight at 37℃ in a 5% CO2 atmosphere. For subcutaneous (s.c.) implantation, a small skin incision was made at the nape of the neck to allow insertion of an 11-gauge tumor implant trocar. The trocar containing the hollow fiber capsules was inserted through the subcutaneous tissue. Generally, each mouse received 4 hollow fiber capsules, each containing 4 different cell lines. Therapy was carried out for 8 days.
The anti-tumor activity was evaluated using the MTT assay. The fibers were placed into a 2 ml of fresh, pre-warmed (37℃) culture medium/35 mm dish, and allowed to equilibrate for 30 min at 37℃. The fibers were then stained with MTT solution (MTT 1 mg/ml) and washed twice with PBS containing 2.5% protamine sulfate. The formazan extracted from the fiber was dissolved with DMSO, transferred to individual wells in 96-well plates, and then accessed for optical density at a 540 nm. The evaluation term was 9 days.
6) Xenografts assay1-2×107 cells were s.c. injected in the right flank of 6 week old male BALB/C nu/nu mice. Tumor bearing mice were subdivided into groups of eight to ten mice. Therapy was initiated 5 days after the development of the tumor volume, when the mean tumor volume was 100~200 mm3. The evaluation term was 25 days.
The tumor volumes were calculated as 1/2(long length × short length2), and expressed in mm3. The tumor growth inhibition was calculated as inhibition (%)=100-[100 × (mean size of treated tumors/mean size of control tumors)].
Any statistically significant difference between the mean values was estimated using Microsoft Excel and an independent t-test for unequal variances.
RESULTSA spectrum of various tumor cell lines was screened for in vivo anti-tumor agents' sensitivity using the xenografts (Table 1) and hollow fiber assays (Table 2). Each cell line was operationally defined to be sensitive to tested anti-tumor agents if the tumor regression or inhibition rate was increased to 50% or more of the control at the concentrations used.
Table 1 presents the results of the xenografts assay. Eight tumor cell lines were sensitive to paclitaxel; SK-OV-3, SK-MEL-2, KB, A549, A431, PC-3, MDA-MD-231 and Hep-G2. Of these, KB and Hep-G2 were found to be highly sensitive cell lines, with inhibition rates of 76 and 77%, respectively. In contrast, a tumor cell line with low sensitivity was SK-MEL-2, with an inhibition rate of 50%. There were 9 adriamycin-sensitive tumor cell lines; SiHa, Capan-2, A431, Colo-230, MKN 45, MKN 28, NCI-H209, FaDu and Hep-G2. Of these, FaDu was found to be a highly sensitive cell line, with an inhibition rate of 80%. In contrast, the SiHa was found to have low sensitivity, with an inhibition rate of 50%. There were 9 5-Fluorouracil-sensitive tumor cell lines; MCF-7, SK-MEL-2, KB, HCT 116, WiDr, MKN 45 MKN 28, MDA-MD-231 and NCI-H209. In case of 5-fluorouracil, the highly sensitive tumor cell lines were MCF-7, SK-MEL-2 and MKN 28, with 89, 90 and 88%, inhibition rates, respectively. Capan-2 exhibited strong resistance against 5-fluorouracil, with 0% inhibition. There were 4 mitomycin-sensitive tumor cell lines; SK-OV-3, HeLa, MCF-7 and SK-MEL-2. The most highly sensitive tumor cell line against mitomycin was SK-OV-3, with 87% inhibition.
Table 2 presents the results of the hollow fiber assay. There were 10 paclitaxel-sensitive tumor cell lines; SK-OV-3, SK-MEL-2, KB, A549, Capan-2, A431, MKN 28, PC-3, MDA-MD-231 and Hep-G2. Most of these, which were sensitive in the xenografts assay, were also sensitive in the hollow fiber assay. Of these, KB, A549 and Hep-G2 exhibited strong activity, with inhibition rates of 86, 87 and 77%, respectively. Specifically, Capan-2, which was evaluated as a resistant cell line, with an inhibition rate of 47% in the xenografts assay, exhibited an inhibition rate of 51% in the hollow fiber assay. Therefore, Capan-2 was evaluated as being a sensitive cell line against paclitaxel in the hollow fiber assay. However, Capan-2 was shown to be a resistant cell line in the xenografts assay, but the variation in the inhibition rate between the two assays was not significant. There were 9 adriamycin-sensitive tumor cell lines; SK-OV-3, SiHa, Colo-230, MKN 45, MKN 28, NCI-H209, FaDu and Hep-G2. FaDu was found to be a highly sensitive tumor cell line, with an inhibition rate of 66% in the xenografts assay, with 80% inhibition. In contrast, SiHa was shown to be a low sensitivity cell line, with 50% inhibition, which exhibited an inhibition rate of 60% in the xenografts assay. SK-OV-3, which exhibited resistance in the xenografts assay, was detected as a sensitive cell line in the hollow fiber assay, but the variation in the inhibition rate between the two assays was not significant. The inhibition rates of Capan-2 and A431 were 57 and 64%, respectively, in the xenografts assay, but these were 27 and 20%, respectively, in the hollow fiber assay. From these results, the inhibition rates of Capan-2 and A431 exhibited large differences between the xenografts and hollow fiber assays, but no correlation was detected between the two assays. Nevertheless, most cell lines that were sensitive in the xenografts assay were also sensitive in the hollow fiber assay. There were 9 5-Fluorouracil sensitive tumor cell lines; MCF-7, SK-MEL-2, KB, HCT 116, Capan-2, MKN 45, MKN 28 and NCI-H209. In case of 5-fluorouracil, MCF-7, SK-MEL-2 and MKN 28 were found to be highly sensitive, at 89, 90 and 88%, respectively. Specifically, Capan-2, which exhibited complete resistance, with an inhibition rate of 0%, exhibited strong sensitivity, at 71%. Nevertheless, most cell lines that were sensitive in the xenografts assay were also sensitive in the hollow fiber assay. There were 4 mitomycin sensitive tumor cell lines; SK-OV-3, HeLa and MCF-7. SK-MEL-2, which exhibited sensitivity in the xenografts assay, with resistance with 47% inhibition rates.
Eight out of 20 cell lines were sensitive to paclitaxel in the xenografts and hollow fiber assays, whereas two cell lines, Capan-2 and MKN-28, exhibited different responses against paclitaxel, only showing sensitivity in the hollow fiber assay. When treated with adriamycin, seven cell lines showed sensitivity in both the xenografts and hollow fiber assays, but two, SK-OV-3 and Capan-2, showed different responses. Capan-2 exhibited a considerable degree of variation in its response to the drugs, whereas SK-OV-3, although resistant to adriamycin in the xenografts assay, exhibited only a tiny degree of variation in its response in the hollow fiber assay.
With the 5-fluorouracil, seven cell lines showed sensitivity in both the xenografts and hollow fiber assays, whereas two, WiDr and Capan-2, exhibited considerable degrees of difference in their responses in the two assays. WiDr showed a high sensitivity in the xenografts assay only, and Capan-2 only in the hollow fiber assay.
With the mitomycin C treatment, three cell lines showed sensitivity in both the xenografts and hollow fiber assays, whereas SK-MEL-2 exhibited sensitivity only in the xenografts assay.
Table 3 presents the correlation of the results between the xenografts and hollow fiber assays. In this correlative study, the hollow fiber assay had a sensitivity of 83% and resistance of 92%; the overall correlation for primary screening between the xenografts and hollow fiber assays was 89%.
Our analysis indicates that, at least with respect to the %age inhibition parameter, the xenografts and hollow fiber assays generally yielded similar results in anti-tumor activity screening. However, there was no significant difference in the results between the assay methods.
DISCUSSIONThe process of discovering cancer or other drugs may begin with either empiric screening or rational drug designing. In either case, the necessary steps in drug development require appropriate animal model systems (5~7). Historically, cancer drug discovery and the efforts on their development have relied on in vivo tumor models. In general, programs for cancer drug discovery and development, such as in vitro systems, MTT assay, SRB assay, neutral red assay and clonogenic assay, have constituted the primary screening methods, and have been tested over a period of 50 years (8~12). Additional murine tumor systems, and some of the more recently developed human tumor xenografts systems, have supplemented these primary systems (13~19).
This study was carried out to give a tool of the primary in vivo screen. As the results have indicated, these assay systems showed high correlation with regard to drug-sensitivity and drug-resistance. To obtain these results, various tumor cell lines were used to evaluate tumor growth inhibition with four anti-tumor agents. The anti-tumor agents were selected from a variety of groups, including taxene, anthracycline, cytosine and alkylating agents. The correlation obtained from these results showed that the true positive, or both assays showing sensitivity to the drug in the same tested cell lines, was 86% and that false negative, or the same cells that showed sensitivity in xenografts assay now showing resistance in hollow fiber assay, was 10%. The hollow fiber assay is a unique in vivo model, which includes in vitro evaluation, with final evaluation using the MTT assay, which is a colorimetric enzyme assay. In contract, the evaluation of the xenograft assay is measured via the tumor volume (size) or weight. The end points between the hollow fiber and xenografts assays are rather different. Five false negatives were found when using the hollow fiber assay in this experiment. Adriamycin, 5-fluorouracil and mitomycin C were detected as false negatives with Capan-2 and A431, WiDr and MDA-MD-231, and SK-MEL-2, respectively. The cause of the different false negatives with the hollow fiber assay was probably due to differences in the pharmacokinetic, evaluation term and end point of the experiment. The pharmacokinetic action is probably different in the hollow fiber membrane, that is, the fiber wall constitutes an artificial barrier that separates the tumor cells from their surroundings. Thus, some differences in this environment could have occurred due to the drug sensitivity in the hollow fiber assay. Within the hollow fiber, the tumor growth is limited by the geometric constraint of the fiber wall, causing differences in the evaluation term between the hollow fiber and xenografts assay. The final evaluation was also different. These differences possibly occurred due to the change in the sensitivity to the drug. Although the hollow fiber assay exhibited some disadvantages, it showed accuracy in predicting drugs that are likely to be active in the xenografts assay. Mi and colleagues (20) established growth conditions for HL-60, HUVEC, Ishikawa, KB, Kb-V1, LNCaP, Lul, MCF-7, Mel2, P-388 and SW626 cells implanted at the intraperitoneal and subcutaneous compartments of athymic mice. Five cytotoxic natural products, including ochraceolide A, α-lapachone, 2-(1-hyroxyethyl)naphtha[2,3-b]furan-4,9-quinone, dioscin and 13-methoxy-15-oxozoapathin were tested in their model, along with paclitaxel. These studies illustrated the usefulness of the hollow fiber model in natural product drug discovery programs. In resent reports, alkylating dipeptide melphalanyl-p-L-fluorophenylalanine ethyl ester, achiral seco-cyclopropylindoline analogs and indole-based natural products were newly found using the hollow fiber assay (21~23). In rats, pyridyl cyanoguanidine has also been tested in vivo using a hollow fiber model, with three of the cell lines (24). With this, the hollow fiber assay was able to be used with various experimental animal systems, rather than with xenografts animal only.
However, this study was not intended to replace xenografts assay system, as it does not model the complex interactions and phenomena that occur when tumor cells are growing in and interacting with the host tissues (25). Nevertheless, in our study, the hollow fiber assay showed good correlation with the xenografts assay for drug screening. The high correlation between the hollow fiber and xenografts assays suggests that the former can be used to overcome the disadvantage of the latter system. In future, large randomized prospective studies will be necessary to determine the overall impact of this predictive test for the development of in vivo systems or drug discovery and development.
References1. Hollingshead MG, Alley MC, Camalier RF, Abott BJ, Mayo JG, Malspeis L, et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. PMID: 7603295
2. Hollingshead M, Roberson J, Decker W, Buckheit R Jr, Elder C, Malspeis L, et al. In vivo drug screening applications of HIV-infected cells cultivated within hollow fibers in two physiologic compartments of mice. Antiviral Res. 1995;28:265–279. PMID: 8629818
3. Casciari JJ, Hollingshead MG, Alley MC, Mayo JG, Malspeis L, Miyauchi S, et al. Growth and chemotherapeutic response of cells in a hollow fiber in vitro solid tumor model. J Natl Cancer Inst. 1994;86:1846–1852. PMID: 7990159
4. Sadar MD, Akopian VA, Beraldi E. Characterization of a new in vivo hollow fiber assay model for the study of progression of prostate cancer to androgen independence. Mol Cancer Ther. 2002;1:629–637. PMID: 12479223
5. Jayaraman M, Fox BM, Hollingshead M, Kohlhagen G, Pommier Y, Cushman M. Synthesis of new dihydroindeno [1,2-c] isoquinoline and indenoisoquinolinium chloride topoisomerase I inhibitors having high in vivo anticancer activity in the hollow fiber animal model. J Med Chem. 2002;45:242–249. PMID: 11754595
6. Hu K, Yao X. The cytotoxicity of methyl protoneoracillin (NSC-698793) and gracillin (NSC-698787), two steroidal saponins from rhizomes of Dioscorea collettii var. hypoglauca, against human cancer cells in vitro. Phytother Res. 2003;17:620–626. PMID: 12820229
7. Rockwell S. In vivo-in vitro tumor cell lines: characteristics and limitations as models for human cancer. Br J Cancer Suppl. 1980;4:118–122. PMID: 6932914
8. Borenfreund E, Puerner JA. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/Nr-90). Tissue Culture Meth. 1984;9:7–9.
9. Driscoll JS. The preclinical new drug research program of the National Cancer Institute. Cancer Treat Rep. 1984;68:63–76. PMID: 6692438
10. Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. PMID: 2359137
11. Salmon SE, Hamburger AW, Soehnlen B, Durie BG, Alberts DS, Moon TE. Quantitation of differential sensitivity of human tumor stem cells to anticancer drugs. N Engl J Med. 1978;298:1321–1327. PMID: 77475
12. Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. PMID: 2359136
13. Bellet RE, Danna V, Mastrangelo MJ, Berd D. Evaluation of a nude mouse-human tumor panel as a predictive secondary screen for cancer chemotherapeutic agents. J Natl Cancer Inst. 1979;63:1185–1188. PMID: 91697
14. Gazdar AF, Carney DN, Sims HL, Simmons A. Heterotransplantation of small-cell carcinoma of the lung into nude mice: comparison of intracranial and subcutaneous routes. Int J Cancer. 1981;28:777–783. PMID: 6277800
15. Houghton JA, Taylor DM. Growth characteristics of human colorectal tumors during serial passage in immune-deprived mice. Br J Cancer. 1978;37:213–223. PMID: 629859
16. Neely JE, Ballard ET, Britt AL, Workman L. Characteristics of 85 pediatric tumors heterotransplanted into nude mice. Exp Cell Biol. 1983;51:217–227. PMID: 6873437
17. Ovejera AA, Houchens DP. Human tumor xenografts in athymic nude mice as a preclinical screen for anticancer agents. Semin Oncol. 1981;8:386–393. PMID: 7323810
18. Povlsen CO, Rygaard J. Heterotransplantation of human adenocarcinomas of the colon and rectum to the mouse mutant nude. A study of nine consecutive transplantations. Acta Pathol Microbiol Scand A. 1971;79:159–169. PMID: 4325120
19. Maruo K, Ueyama Y, Inaba M, Emura R, Ohnishi Y, Nakamura O, et al. Responsiveness of subcutaneous human glioma xenografts to various antitumor agents. Anticancer Res. 1990;10:209–212. PMID: 2334129
20. Mi Q, Lantvit D, Reyes-Lim E, Chai H, Zhao W, Lee IS, et al. Evaluation of the potential cancer chemotherapeutic efficacy of natural product isolates employing in vivo hollow fiber tests. J Nat Prod. 2002;65:842–850. PMID: 12088425
21. Gullbo J, Lindhagen E, Bashir-Hassan S, Tullberg M, Ehrsson H, Lewensohn R, et al. Antitumor efficacy and acute toxicity of the novel dipeptide melphalanyl-p-L-fluorophenylalanine ethyl ester (J1) in vivo. Invest New Drugs. 2004;22:411–420. PMID: 15292711
22. Kupchinsky S, Centioni S, Howard T, Trzupek J, Roller S, Carnahan V, et al. A novel class of achiral seco-analogs of CC-1065 and the duocarmycins: design, synthesis, DNA binding, and anticancer properties. Bioorg Med Chem. 2004;12:6221–6236. PMID: 15519165
23. Labarbera DV, Skibo EB. Synthesis of imidazo[1,5,4-de]quinoxalin-9-ones, benzimidazole analogues of pyrroloiminoquinone marine natural products. Bioorg Med Chem. 2005;13:387–395. PMID: 15598560
24. Hovstadius P, Lindhagen E, Hassan S, Nilsson K, Jernberg-Wiklund H, Nygren P, et al. Cytotoxic effect in vivo and in vitro of CHS 828 on human myeloma cell lines. Anticancer Drugs. 2004;15:63–70. PMID: 15090745
25. Berger DP, Fiebig HH, Winterhalter BR, Wallbrecher E, Henss H. Preclinical phase II study of ifosfamide in human tumor xenografts in vivo. Cancer Chemother Pharmacol. 1990;26(Suppl):S7–S11. PMID: 2347054
Table 1Tumor regression effect of various anti-tumor agents in the xenografts model using human tumor cell lines 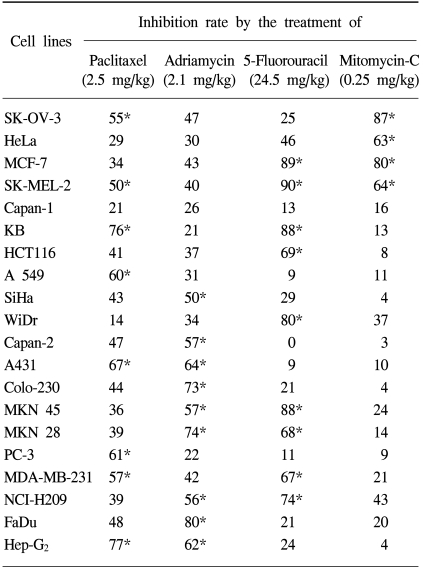
Table 2The effect of various anti-tumor agents on human tumor cell lines cultivated in hollow fibers in mice 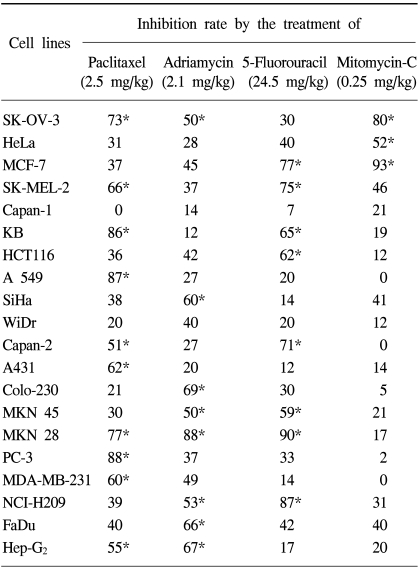
Table 3Correlations of the results exhibited for the Hollow fiber versus the xenografts assay 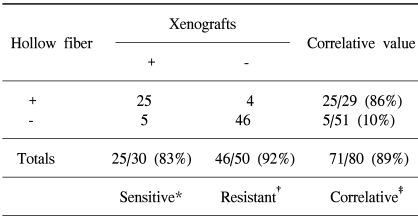 With the t-test, the association between xenografts and hollow fiber result was significant (p<0.05). *Sensitive is defined as the ability to predict positive responses in the xenografts assay, †Resistant is defined as the ability to predict negative responses in the xenografts assay, ‡Correlation was defined as the ability to predict the overall in vivo assay responses. |
|
||||||||||||||||||||||||||||||||||||||