AbstractPurposeThis phase II study investigated whether durvalumab/tremelimumab with proton therapy improves the objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) in heavily treated recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) patients.
Materials and MethodsPatients who previously received more than one chemotherapy, including at least one platinum-based regimen, and who had at least two measurable lesions were enrolled. Patients received 1,500 mg durvalumab intravenously combined with 75 mg tremelimumab intravenously every 4 weeks for four cycles followed by 1,500 mg durvalumab every 4 weeks. After one cycle of the durvalumab/tremelimumab treatment, proton therapy was given with a total dose of 25 Gy in 5 Gy daily fractions to one of the measurable lesions. We also assessed the ORR in the target lesion outside the radiation field to evaluate the abscopal effect.
ResultsThirty-one patients were enrolled between March 2018 and July 2020. With 8.6 months of follow-up, the ORR was 22.6% (7/31), including one complete response and six partial responses. The median OS was 8.4 months (95% confidence interval [CI], 2.5 to 14.3) and the median PFS was 2.4 months (95% CI, 0.6 to 4.2). Among the 23 evaluable patients who completed proton therapy, the ORR was 30.4% (7/23). The median OS was 11.1 months (95% CI, 6.5 to 15.8), and the median PFS was 3.7 months (95% CI, 1.6 to 5.7). Grade 3 or higher adverse events were observed in six patients (19.4%) as follows: anemia (n=1), constipation (n=1), electrolyte imbalances (n=2), hyperglycemia (n=1), and pneumonia (n=1).
IntroductionApproximately 60% of patients with head and neck squamous cell carcinoma (HNSCC) are diagnosed with locally advanced or metastatic disease. They have a low survival rate and many of them will experience locoregional recurrence or distant metastasis [1]. Patients with recurrent or metastatic (R/M) HNSCC generally have a dismal prognosis and poor outcome. Platinum-based doublet chemotherapy with cetuximab as first-line chemotherapy has been considered the standard of care (SoC) since 2007 [2]. However, since the introduction of immune checkpoint inhibitors (ICIs) for cancer treatment, breakthroughs have been made in many solid cancers, including HNSCC. The anti–programmed cell death 1 (anti–PD-1), pembrolizumab with chemotherapy, showed improvement of overall survival (OS) versus cetuximab with chemotherapy in the KEYNOTE-048 trial as a first-line therapy in HNSCC (13.0 vs. 10.7 months; 95% confidence interval [CI], 0.63 to 0.93) [3]. The KEYNOTE-040 trial showed OS improvement with anti–PD-1, pembrolizumab treatment, while the Checkmate-141 trial confirmed better OS with another anti–PD-1, nivolumab, over SoC cytotoxic chemotherapy in HNSCC patients as a second-line therapy [4,5]. Recently, pembrolizumab alone has shown improvements in first-line setting, emphasizing the clinical usefulness of immunotherapy in HNSCC [6]. Nevertheless, the median OS in HNSCC patients still remains in the range of 7.5–8.4 months in R/M setting, thus novel strategies to improve outcomes are needed [5,7].
Durvalumab is a human monoclonal antibody (mAb) of the IgG 1 kappa subclass that blocks the interaction of programmed death-ligand 1 (PD-L1) with PD-1 on T cells and CD80 (B7.1) on immune cells [8]. Tremelimumab is a human IgG2 mAb that targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and completely blocks the interaction of human CTLA-4 with CD80 and CD86, resulting in increased release of cytokines [9]. Double blockade of CTLA-4 and PD-L1/PD-1 pathways could have additive or synergistic effects [10]. The combination of durvalumab and tremelimumab showed better efficacy than monotherapy in non-small cell lung cancer [11]. However, in metastatic HNSCC, durvalumab plus tremelimumab combination did not show better OS than durvalumab monotherapy or SoC in phase III EAGLE study [12]. It also did not prove superior objective response rate (ORR) compared with durvalumab monotherapy in phase II CONDOR trial [13].
Radiation therapy (RT) is a main treatment modality for HNSCC. RT causes DNA damage, which leads to direct tumor cell death [14]. It might enhance the immunologic response by altering the microenvironment within the irradiated field, increasing tumor antigen release, and having an “abscopal effect” at distant metastatic sites [15]. The abscopal effect refers to the ability of local RT to induce an anti-tumor response throughout the body in the out-field of RT. Mole [16] first introduced the abscopal effect in the early 1950s when he coined the term abscopal after observing a clinical response to irradiation at distant sites that were not treated with RT.
Proton therapy is a type of RT that uses proton particles rather than X-rays. By exploiting the Bragg peak phenomenon of protons, which allows extremely targeted delivery of RT, the dose to surrounding normal tissues can be reduced compared to X-ray treatment [17]. Thus, proton therapy can reduce the toxicity of RT in HNSCC patients. In addition, proton therapy may have immunomodulatory benefits by lowering RT-induced lymphopenia and avoiding immunosuppression in addition to other various biologic effects [18,19].
In this phase II study, we investigated whether durvalumab plus tremelimumab as an immunotherapy combined with proton therapy improved the treatment outcomes of patients with heavily treated R/M HNSCC via a potential synergic effect through combined ICIs with proton therapy.
Materials and Methods1. Study designThe study was designed as a single-arm, prospective phase II study under the ethical principles of the Declaration of Helsinki and the Korea Good Clinical Practice guidelines. The study protocol was approved by the Institutional Review Board of Samsung medical center (NCT03450967). Written informed consent was obtained from the participants before study enrollment. The subjects were willing and able to comply with the protocol for the duration of the study, including undergoing treatment, scheduled visits, and follow-up examinations.
Patients received 1,500 mg durvalumab plus 75 mg tremelimumab via intravenous infusion every 4 weeks up to four cycles, followed by 1,500 mg durvalumab monotherapy via intravenous infusion every 4 weeks until the point of disease progression or intolerable toxicity.
After one cycle of durvalumab plus tremelimumab and before the second immunotherapy cycle, patients received a proton therapy dose of 5 GyE×5 fractions (Fig. 1). The robust optimization was performed using RayStatation (treatment planning system) with 3 mm of set-up uncertainty and 3.5% of range uncertainty. Basically, the largest area among the lesions was determined as the irradiation target. If the gross target volume (GTV) includes or is located within 1 cm from the carotid artery, the target will be changed to the next largest one. If the next target is not feasible because of the same reason, the delineation of GTV will be modified to allow partial irradiation. The GTV will be reduced by at least 1 cm far from the carotid artery to minimize the irradiated dose to the carotid artery.
2. PatientsBetween March 2018 and July 2020, 31 patients with R/M HNSCC were enrolled at (anonymized for review). Eligible patients were aged ≥ 18 years, had at least two measurable lesions, and had histologically proven HNSCC. The PharmDx assay (Dako, Carpinteria, CA) involved staining with an anti–PD-L1 22C3 mouse monoclonal primary antibody and was performed using the EnVision FLEX visualization system (Agilent, Santa Clara, CA) on an Autostainer Link 48 system (Dako) along with positive and negative controls, as per the manufacturer’s instructions. Patients were required to have a history of more than one previous systemic chemotherapy including at least one platinum-based regimen. The last day of anticancer therapy (chemotherapy, immunotherapy, endocrine therapy, targeted therapy, biologic therapy, tumor embolization, and monoclonal antibodies) was allowed at least 30 days prior to the first dose of the study drugs. The specific inclusion criteria and exclusion criteria are described in the Supplementary Materials.
3. Outcome measurementsThe primary endpoint was the ORR. Secondary endpoints included the duration of response (DoR), progression-free survival (PFS), and OS. The endpoint evaluations were performed by investigator assessments according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1. The tumor assessments were performed by computed tomography or magnetic resonance imaging every 8 weeks until disease progression. Tumor responses were measured in both the irradiated lesions (in-field target) and non-irradiated lesions (out-field target) to determine if an abscopal effect occurred. Adverse events (AEs) were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) ver. 4.0.
4. Statistical analysisAccording to Simon’s two-stage optimal design (power of 90% and one-sided alpha of 0.05), this study required a total of 31 evaluable patients. The sample size was calculated to reject 10% response rate in favor of a target response rate of 35% with a significance level of 0.05 and power of 90%. In the first stage, 11 patients were enrolled. As two or more of these patients showed an objective response, the study proceeded to the second stage. In the second stage, at least 16 additional patients were enrolled for minimum 27 patients (a total of 31 patients). Among the total 31 evaluable patients, six or more objective responses were considered necessary for further evaluation of the treatment regimen in the R/M HNSCC group.
Data are presented as the number (%) for categorical variables and descriptively summarized as proportions and medians. Kaplan-Meier estimates were used in the analysis of all time to event variables and the 95% confidence interval for the median time to event was computed. Statistical significance was considered at p < 0.05 and all tests were two-sided. All statistical analyses were conducted using IBM SPSS statistics ver. 27 (IBM Corp., Armonk, NY).
Results1. Patient characteristicsThe patient demographics are shown in Table 1. The median age was 59 years and 81% of the patients were male. The patient cohort consisted of six patients with oral cavity cancer, five with oropharyngeal cancer, four with hypopharyngeal cancer, four with laryngeal cancer, and 12 patients with other cancers such as nasal cancer, ear canal cancer, and lacrimal sac cancer. All had R/M HNSCC with a history of a median of two (range of 1–7) prior systemic chemotherapy. Patients who had received immunotherapy were not included. Eighty-seven percent of the patients had a Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1 and 13% had an ECOG PS of 2. According to the patient inclusion criteria, ECOG PS of 0 or 1 patients were eligible for recruitment. However, at the beginning of the study, some patients were evaluated as ECOG PS of 2 due to disease progression. The most common site of distant metastasis was the lungs (36%) followed by bone (19%), brain (10%), and liver (10%). All but one patient had a history of RT (median, 2; range, 1 to 9) and 68% (21 of 31) of patients received concurrent chemoradiation therapy. The PD-L1 expression was assessed in 25 of the 31 patients and eight patients had a PD-L1 expression ≥ 1%.
2. EfficacyThe data cutoff date was 31 July 2021 and the median follow-up duration was 8.6 months (range, 1.4 to 30.0). In the intention to treatment analysis, the ORR was 22.6% (7 of 31), which was determined by responses in the out-field lesion. The disease control rate was 41.9% (13 of 31). Among the 31 patients who received one cycle of immunotherapy, three patients experienced disease progression and withdrew from the study. Another three patients dropped out because of their poor general condition. Twenty-five patients underwent proton therapy, one of whom experienced disease progression and one of whom did not complete proton therapy due to poor general condition. Twenty-three patients could completed proton therapy. The median number of cycles of immunotherapy was 3 (range, 1 to 43). The median DoR was 16.5 months (range, 3.7 to 29.3). The median PFS was 2.4 months (95% CI, 0.6 to 4.2) and the median OS was 8.4 months (95% CI, 2.5 to 14.3) (Fig. 2A and B) in total 31 patients. Among the 23 evaluable patients who completed proton therapy, the ORR, out-field response rate, was 30.4% (7 of 23) and the response rate of the proton irradiated lesion was 65.2% (15 of 23) (Table 2). The details of the irradiated lesions and out-field targets are presented in Table 3. The median OS was 11.1 months (95% CI, 6.5 to 15.8), and the median PFS was 3.7 months (95% CI, 1.6 to 5.7) in 23 patients who completed proton therapy. Four of the 31 patients were still undergoing treatment at the data cutoff date. Among these four patients, one had a complete response, two had a partial response, and one had stable disease. A detailed summary of the patients’ treatment responses are described in the swimmer plot (Fig. 3). Kaplan-Meier plots comparing the OS and PFS of responders and non-responders are additionally shown in the S1 Fig.
3. SafetySafety analysis was performed in patients who received at least one dose of immunotherapy. The AEs are shown in Table 4. The median number of cycles was 3 for durvalumab (range, 1 to 43) and 3 for tremelimumab (range, 1 to 4). Treatment-related AEs (TRAEs) for any grade were observed in 25 patients (25/31, 80.6%). Overall, the most common TRAEs of any grade were electrolyte imbalances (19.6%), skin rashes (19.6%), anorexia (16.1%), and nausea (16.1%). Grade 3 or higher AEs were observed in six (19.4%) patients as follows: anemia (n=1), constipation (n=1), electrolyte imbalance (n=2), hyperglycemia (n=1), and elevated amylase and pneumonia (n=1). Two grade 4 AEs, namely hyponatremia and hypercalcemia, were observed, both of which were related to disease progression. Two immune-related AEs were noted: grade 2 hypothyroidism and grade 4 hyperglycemia. The patient with grade 4 hyperglycemia was diagnosed with insulin-dependent diabetes mellitus and started insulin treatment. No treatment-related deaths occurred during the trial (Table 4).
DiscussionIn this study, durvalumab plus tremelimumab combined with proton therapy resulted in an ORR of 22.6% and tolerable safety profiles in previously heavily treated patients with R/M HNSCC. Furthermore, an ORR of 30.4% was observed in the out-field of RT irradiated patients, suggesting that proton therapy combined with ICIs may be more effective. Systemic abscopal effect can be described as inducing tumor regression of out-of-RT field after localized RT mediated by the effects of radiation on the immune system. Several studies have confirmed that synergistic immune effect or abscopal effect of combining RT and immunotherapy [15,20]. Recently, proton therapy has emerged as a promising treatment option for anticancer treatment, and its clinical benefits are well-known for systemic tumor response and immunogenic potential in preclinical and clinical evidence [19]. Although the abscopal effect with proton therapy has primarily been reported in case reports or preclinical data, our prospectively designed study can provide additional evidence of an abscopal effect [21]. Our study is considered the first prospective clinical trial to confirm the abscopal effect resulting from the combination of immunotherapy and proton therapy in head and neck cancer.
To date, the optimal RT dose and fractions required for sensitization to immunotherapy have not been established. A few preclinical and clinical reports have suggested that hypo-fractionated regimens (e.g., 6 Gy×5 fractions or 8 Gy×3 fractions) are more effective than conventional fractionation, although the underlying mechanisms are unclear [22]. The optimal timing of combination RT and immunotherapy has also not been established, and it is unclear if RT should be given concurrently or sequentially with ICIs. In our study, proton therapy comprising 5 GyE×5 fractions was administered after one durvalumab and tremelimumab treatment cycle. We did not find lymphopenia of grade 3 or higher before the second immunotherapy after proton therapy (S2 and S3 Tables). Unfortunately, of the 31 patients enrolled, only 25 patients (80.6%) received the planned proton therapy. The other six patients (19.4%) did not receive proton therapy because of rapid deterioration of PS caused by early tumor progression. In addition, the response could not be assessed in two patients who did receive proton treatment due to early progression or deterioration of PS just after the completion of proton therapy. Finally, 25% (8/31) of patients experienced progression. Given the rapid nature of tumor progression in heavily pretreated R/M HNSCC, we suggest that RT should be delivered as early in the cycles of ICI therapy as possible to maximize the anti-tumor effects of combined ICI and RT treatment. Several trials have suggested that receiving ICIs immediately after radiotherapy might result in better clinical outcomes [23–25]. In phase II trial stereotactic body radiation therapy (SBRT) prior to pembrolizumab in non–small cell lung cancer patients showed better ORR and PFS than pembolizumab alone group although no statistically significant difference was found [26]. However, the COSINR phase I trial evaluated concurrent or sequential ipilimumab, nivolumab, and SBRT in patients with stage IV non–small cell lung cancer and found no significant differences in two different timing schedules (median PFS was 5.9 months in the sequential arm and 6.2 months in the concurrent arm) [27]. Furthermore, the recent randomized phase II trial of nivolumab with SBRT versus nivolumab alone in metastatic HNSCC showed no significant differences in ORR (34.5% [95% CI, 19.9% to 52.7%] vs. 29.0% [95% CI, 16.1% to 46.6%]; p=0.86) [28]. Given the conflicting results from previous studies, the role of combinational local radiotherapy in HNSCC should be further evaluated. In our study, proton therapy with 5 Gy×5 fractions after one cycle of ICI therapy was appropriate considering the response rate and tolerable AEs. However, further investigation is required to define the optimal schedule and dose of RT.
The use of dual ICIs is a relatively new treatment approach for patients with advanced stage cancer [29,30]. The combination of durvalumab and tremelimumab was investigated in R/M HNSCC patients. In a phase II CONDOR randomized clinical trial of R/M HNSCC patients, durvalumab plus tremelimumab treatment yielded a median OS (95% CI) of 7.6 (4.9–10.6) months compared to the median OSs of durvalumab monotherapy and tremelimumab monotherapy groups of 6.0 (4.0–11.3) months and 5.5 (3.9–7.0) months, respectively [13]. However, the combination of durvalumab and tremelimumab did not show improvement over durvalumab monotherapy in EAGLE, which is a randomized phase III study [12]. The median OS was 7.6 (6.1–9.8) months for durvalumab, 6.5 (5.5–8.2) months for durvalumab plus tremelimumab, and 8.3 (7.3–9.2) months for the SoC group. An ORR of 18.2% was reported in the EAGLE study, which is slightly lower than our ORR of 22.6%. Our median OS of 8.4 months and ORR of 22.6% are encouraging considering that our patients were more heavily treated (median of two rounds of prior chemotherapy in our study versus one in the EAGLE study) and had a poorer PS than patients in the EAGLE study. In this study, the median number of immunotherapy cycles was 3 (range, 1 to 43) and grade 3/4 TRAEs occurred in six patients (19.4%). This incidence of grade 3/4 TRAEs is slightly higher than to that seen in other studies of durvalumab plus tremelimumab (12.3% and 16.3% in the CONDOR and EAGLE studies, respectively). These prior studies suggested that the combination of durvalumab and tremelimumab might not increase the risk of grade 3/4 TRAEs compared to durvalumab alone.
This study had several limitations. Although tumor response was observed in out-field tumor lesions, a single-arm study without a control group makes it difficult to evaluate the true abscopal effect and whether proton therapy can enhance treatment efficacy. Secondly, since this study allowed enrollment for patients with paranasal sinus cancer, which is a cancer type usually excluded in most clinical trials, the patient population was heterogeneous. Furthermore, the primary tumor site varied among the patients and the evaluation of the abscopal effect according to the primary site is challenging due to the small number of patients for each subgroup. Nevertheless, we observed comparable response to treatment despite heterogeneous primary tumor locations.
In conclusion, this pilot trial of durvalumab plus tremelimumab treatment combined with proton therapy showed favorable efficacy and manageable AEs in heavily pretreated R/M HNSCC patients, suggesting that RT and ICI therapy may exhibit good anti-tumor activity. Further randomized trials are needed to confirm our findings.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study was conducted in accordance with the Declaration of Helsinki and the Korea Good Clinical Practice guidelines. The study protocol was approved (#2017-09-026, NCT03450967) by the Institutional Review Board of Samsung Medical Center (Seoul, Korea). All patients provided written informed consent prior to participation. Author Contributions Conceived and designed the analysis: Oh D, Ahn MJ. Collected the data: Kim H, Park S, Jung HA, Lee SH, Park K, Ahn YC, Oh D, Ahn MJ. Contributed data or analysis tools: Kim H, Oh D, Ahn MJ. Performed the analysis: Kim H, Park S, Jung HA, Lee SH, Park K, Ahn YC. Wrote the paper: Kim H, Oh D, Ahn MJ. AcknowledgmentsThis study was funded by AstraZeneca, and we thank AstraZeneca for their support of the study.
Fig. 1Study design. CTx, chemotherapy; fx, fraction; HNSCC, head and neck squamous cell carcinoma; Q4W, every 4 weeks. 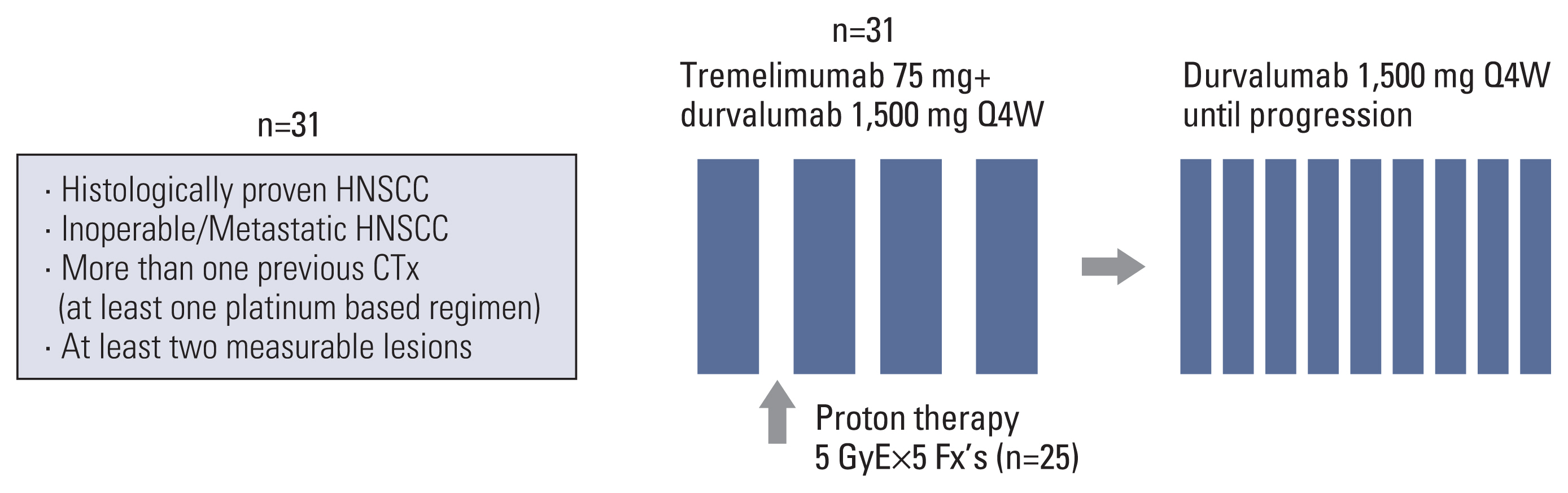
Fig. 3Survival swimmer plot. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease. 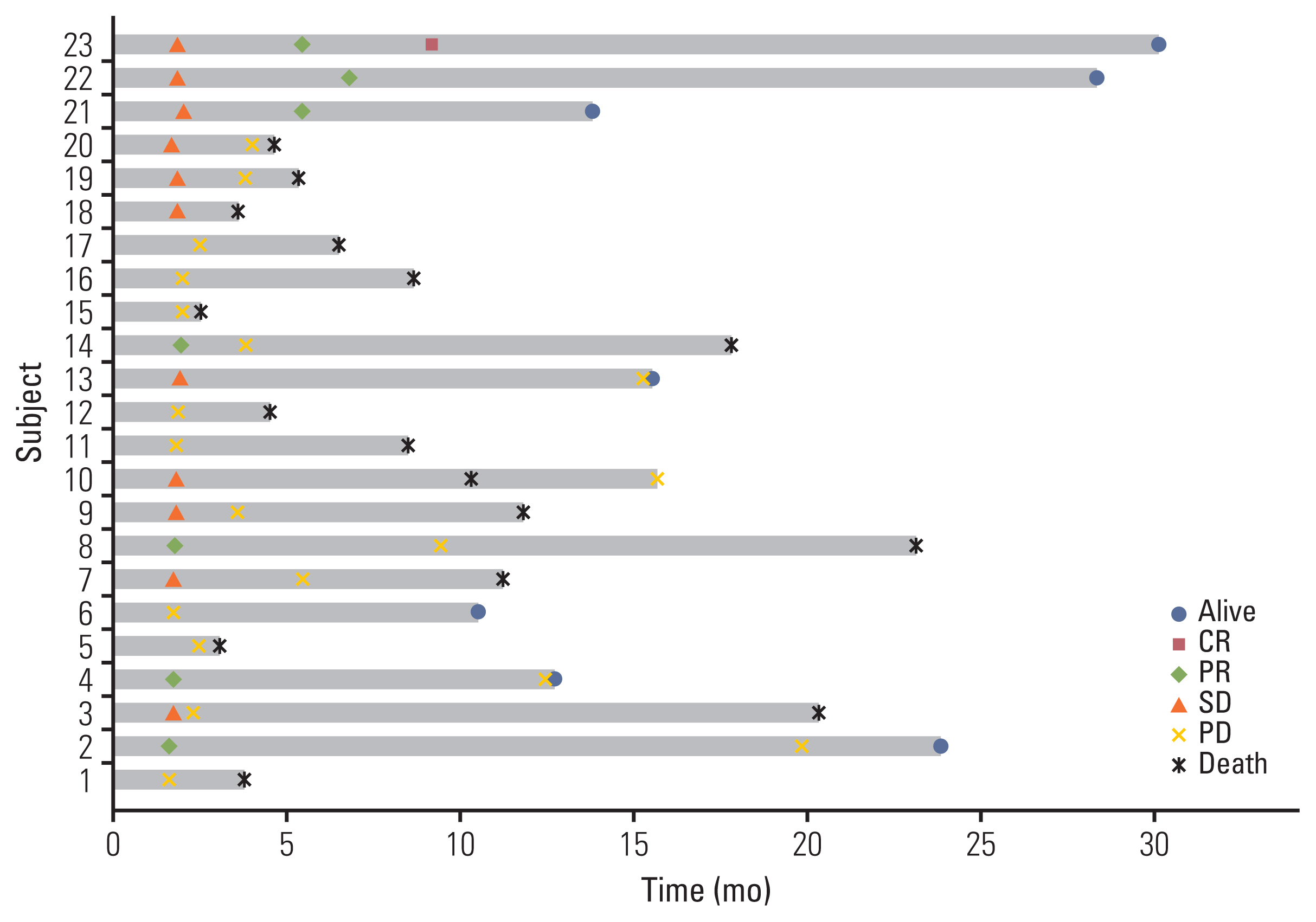
Table 1Patient characteristics
Table 2Objective responses of the patients who were evaluable following proton therapy
Table 3In-field target and out-field target of patients who received proton therapy (n=25) Table 4Incidence of treatment-related adverse events References1. Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21:Suppl 7vii252–61.
2. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27.
3. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28.
4. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
5. Cohen EE, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67.
6. Burtness B, Rischin D, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40:2321–32.
7. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
8. Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3:1052–62.
9. Tarhini AA, Kirkwood JM. Tremelimumab (CP-675,206): a fully human anticytotoxic T lymphocyte-associated antigen 4 monoclonal antibody for treatment of patients with advanced cancers. Expert Opin Biol Ther. 2008;8:1583–93.
10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
11. Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308.
12. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31:942–50.
13. Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5:195–203.
15. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88.
17. Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50:135–42.
18. Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–35.
19. Lee HJ Jr., Zeng J, Rengan R. Proton beam therapy and immunotherapy: an emerging partnership for immune activation in non-small cell lung cancer. Transl Lung Cancer Res. 2018;7:180–8.
20. Zhuang H. Abscopal effect of stereotactic radiotherapy combined with anti-PD-1/PD-L1 immunotherapy: mechanisms, clinical efficacy, and issues. Cancer Commun (Lond). 2020;40:649–54.
21. Brenneman RJ, Sharifai N, Fischer-Valuck B, Hassanzadeh C, Guzelian J, Chrisinger JS, et al. Abscopal effect following proton beam radiotherapy in a patient with inoperable metastatic retroperitoneal sarcoma. Front Oncol. 2019;9:922.
22. Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–24.
23. Goto T. Radiation as an in situ auto-vaccination: current perspectives and challenges. Vaccines (Basel). 2019;7:100.
24. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC: update from PACIFIC. J Thorac Oncol. 2020;15:288–93.
25. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50.
26. Theelen W, Chen D, Verma V, Hobbs BP, Peulen HM, Aerts J, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9:467–75.
27. Bestvina CM, Pointer KB, Karrison T, Al-Hallaq H, Hoffman PC, Jelinek MJ, et al. A phase 1 trial of concurrent or sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV NSCLC study. J Thorac Oncol. 2022;17:130–40.
28. McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39:30–7.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||