AbstractPurposeSeveral previous studies and case reports have reported ethanol-induced symptoms in patients receiving anticancer drugs containing ethanol. Most docetaxel formulations contain ethanol as a solvent. However, there are insufficient data on ethanol-induced symptoms when docetaxel-containing ethanol is administered. The primary purpose of this study was to investigate the frequency and pattern of ethanol-induced symptoms during and after docetaxel administration. The secondary purpose was to explore the risk factors for ethanol-induced symptoms.
Materials and MethodsThis was a prospective, multicenter, observational study. The participants filled out ethanol-induced symptom questionnaire on the day of chemotherapy and the following day.
ResultsData from 451 patients were analyzed. The overall occurrence rate of ethanol-induced symptoms was 44.3% (200/451 patients). The occurrence rate of facial flushing was highest at 19.7% (89/451 patients), followed by nausea in 18.2% (82/451 patients), and dizziness in 17.5% (79/451 patients). Although infrequent, unsteady walking and impaired balance occurred in 4.2% and 3.3% of patients, respectively. Female sex, presence of underlying disease, younger age, docetaxel dose, and docetaxel-containing ethanol amount were significantly associated with the occurrence of ethanol-induced symptoms.
IntroductionSome intravenous anticancer drugs contain ethanol as a solvent to increase their solubility. Widely used chemotherapy formulations containing ethanol include paclitaxel, docetaxel, cabazitaxel, gemcitabine, and etoposide [1]. Several previous studies and case reports have reported ethanol-induced symptoms in patients receiving anticancer drugs containing ethanol [2–5]. Docetaxel is used alone or in combination with chemotherapy for various types of cancer, such as breast, prostate, lung, stomach, and head and neck cancers. Most docetaxel formulations contain ethanol as a solvent. In 2014, the U.S. Food and Drug Administration (FDA) warned that the intravenous chemotherapy drug docetaxel contains ethanol, which may cause patients to experience alcohol intoxication or feel drunk during and after treatment [6]. The ethanol-induced symptoms during or after docetaxel administration are temporary and difficult to distinguish from symptoms caused by adverse events of anticancer drugs, the patient’s underlying disease, and/or concomitant medications. However, since there are only case reports or small-scale studies on this issue, there are insufficient data on the frequency, the pattern of occurrence, and predisposing factors of ethanol-induced symptoms when docetaxel-containing ethanol is administered. Based on this background, we planned a prospective observational study on ethanol-induced symptoms in patients receiving docetaxel formulations containing ethanol. In addition, since ethanol-induced symptoms may appear even at lower blood ethanol levels in Asians, this study on Korean patients was expected to provide more meaningful data.
Materials and Methods1. Study objectives and designThe primary purpose of this prospective, multicenter, observational study was to investigate the frequency and pattern of ethanol-induced symptoms during and after docetaxel administration. The secondary purpose was to exp-lore the risk factors for ethanol-induced symptoms. A self-symptom questionnaire (S1 Fig.) was developed to evaluate ethanol-induced symptoms based on the literature [3,7]. The participants completed an ethanol-induced symptom questionnaire on the day of chemotherapy (before docetaxel injection, during docetaxel injection, and within 30 minutes from completion of docetaxel injection) and the following day (24 hours after completion of docetaxel injection). The investigator or co-investigator permitted phone surveys the following day. There were no treatment guidelines in the study protocol, such as the administration of chemotherapy, including docetaxel. Therefore, the brand name and administration method of docetaxel were determined by the investigators of each institution. This study was conducted from June 2020 to February 2021 at 21 institutions in South Korea.
2. Sample size determinationPrior systematic studies on ethanol-induced symptoms caused by the administration of ethanol-containing docetaxel are lacking. The occurrence rate of ethanol-induced symptoms was 33.3% in a study of patients treated with ethanol-containing gemcitabine [3]. Therefore, the occurrence rate of ethanol-induced symptoms during or after docetaxel administration was assumed to be approximately 30%. The number of subjects was calculated with an accuracy of 95% confidence intervals and within 8% of the width of the confidence interval [8,9]. A total of 505 calculated patients were included, and 562 subjects were required, considering a dropout rate of 10%. Because of the delayed subject enrollment, the study was closed after 458 enrollments. The width of the confidence interval for the primary endpoint after early study discontinuation was 9.37%. Since there was no remarkable difference between the confidence interval width at the time of the study design and the confidence interval width at the early end of the study, we closed the study subject registration and analyzed the data.
3. Study patientsThis study included patients who (1) were aged 19 years or older, (2) were diagnosed with cancer, (3) received chemotherapy including docetaxel (above 50 mg/m2) that contained ethanol, (4) had an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, (5) could read and understand documents written in Korean, and (6) signed the informed consent form. The exclusion criteria were as follows: (1) could not read and write the survey documents, (2) with significant neurological or psychiatric disorder, (3) metastasis to the central nervous system, (4) active hepatitis, (5) significant anemia (hemoglobin < 9 g/dL), (6) abnormal liver function test results (aspartate aminotransferase, alanine aminotransferase, or total bilirubin ≥ two times or more than the upper limits of the normal range), (7) received chemotherapeutic drug(s) containing ethanol except docetaxel, (8) consumed alcohol within 24 hours before docetaxel administration, (9) intake of hypnotics or sedatives within 24 hours before docetaxel administration, (10) pregnant or breastfeeding, (11) participated in another clinical trial or had a plan to participate during the study period, (12) and deemed not suitable to participate in the study as judged by the investigator.
4. Data collectionIn addition to the survey, the following data were collected: (1) sex and age, (2) detailed history of alcohol consumption; (3) height, body weight, and body mass index; (4) diagnosis of cancer; (5) ECOG performance status; (6) comorbidities; (7) current medications; (8) detailed data of docetaxel injection: brand name, dose, amount of ethanol administration; and (9) detailed data of intravenous solutions for infusion before, during, and after the docetaxel injection. In addition, the blood alcohol content was calculated using a simplified version of the Widmark formula [10]. The ethanol amounts contained in the docetaxel formulation according to the manufacturer were calculated based on the data summarized in S2 Table [6].
5. Statistical analysisEthanol-induced symptoms were considered to have occurred if the number of symptoms increased during or after docetaxel administration compared to before administration. Continuous variables were presented as descriptive statistics (subject number, mean, and standard deviation). For categorical variables, the numbers of patients and percentages (%) were presented. The ethanol-induced symptoms were presented as 95% Clopper-Pearson’s exact confidence interval of occurrence rate, and the occurrence rate differences were analyzed using the chi-square test or Fisher’s exact test for risk factors. The overall occurrence of ethanol-induced symptoms was analyzed using simple logistic regression, and the odds ratios, 95% Wald confidence intervals, and p-values were presented as risk factors. In addition, multiple logistic regression analysis was performed to identify risk factors affecting the overall occurrence of ethanol-induced symptoms, and the odds ratio, 95% Wald confidence interval, and p-value were presented. The results of all variables, including the outcomes, were rounded to the second decimal place, and the p-value was presented to the fourth decimal place. Statistical significance was set at p < 0.05, and all p-values corresponded to the two-sided significance tests. Statistical analysis was performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results1. Baseline characteristics of the study patientsA total of 458 patients were enrolled, and the data from 451 patients were analyzed, excluding seven patients who dropped out. The baseline characteristics of the patients are shown in Table 1. The median age was 54.0 years (range, 23 to 86 years). Seventy-four patients were male (16.4%), and 377 were female (83.6%). The median height, weight, and body mass index were 159.7 cm, 59.4 kg, and 23.4 kg/m2. A total of 412 patients (91.4%) did not drink alcohol. Two-hundred-and-twelve patients (47.0%) had at least one underlying disease. When classified according to the purpose of chemotherapy, 197 patients (43.7%) received adjuvant chemotherapy, 165 patients (36.6%) received neoadjuvant chemotherapy, and 89 patients (19.7%) received palliative chemotherapy. A total of 230 patients (51.0%) were treated with combination therapy, and 221 patients (49.0%) were treated with docetaxel monotherapy. The anticancer drugs included in the combination therapy are summarized in S3 Table. The detailed docetaxel administration data are shown in Table 2 and S4 Table. The doses of ethanol administered per hour are shown in S5 Table.
2. Descriptive analysis for ethanol-induced symptomsEthanol-induced symptoms increased in 200 of 451 patients during or after docetaxel administration compared to before administration. Therefore, the overall occurrence rate of ethanol-induced symptoms was 44.3% (95% confidence interval, 39.7 to 49.1). The ethanol-induced symptoms were classified into category 1 (dizziness, difficulty speaking, unsteady walking, impaired balance, mood swings, and slower reactions) and category 2 (facial flushing, headache, nausea, palpitation, and blurred vision). Category 1 symptoms were derived from a previous study [3], and category 2 symptoms were derived from the symptoms of acute ethanol intoxication [7]. The occurrence rates for each ethanol-induced symptom category were 21.3% in category 1 (96/451 patients) and 37.7% in category 2 (170/451 patients). In the analysis of the individual symptom occurrence (Fig. 1), the occurrence rate of facial flushing was the highest in 19.7% (89/451 patients), followed by nausea in 18.2% (82/451 patients), and dizziness in 17.5% (79/451 patients). The occurrence of ethanol-induced symptoms by time point was the highest at “24 hours after completion of docetaxel injection” (28.8%, 130/451 patients), followed by “during docetaxel injection” (20.2%, 91/451 patients), and “within 30 minutes from completion of docetaxel injection” (18.4%, 83/451 patients). Analysis of the occurrence rate of ethanol-induced symptoms during administration revealed that dizziness occurred most frequently in 8.9% (40/451 patients), followed by nausea in 6.7% (30/451 patients), palpitation in 5.1% (23/451 patients), and facial flushing 4.9% (22/451 patients). Within 30 minutes after the end of the administration, dizziness occurred most frequently in 7.8% (35/451 patients), followed by nausea in 6.2% (28/451 patients), flushing in 4.7% (21/451 patients), and headache with 3.8% (17/451 patients) (Table 3).
3. Risk factors for the occurrence of ethanol-induced symptomThe overall occurrence rate of ethanol-induced symptoms was 32.4% (24/74 patients) in males and 46.7% (176/377 patients) in females (p=0.024). The overall occurrence rate of ethanol-induced symptoms was 37.7% (80/212 patients) in patients with underlying disease(s) and 50.2% (120/239 patients) in patients without underlying disease(s) (p=0.008). However, there was no statistically significant difference in the occurrence of ethanol-induced symptoms according to alcohol drinking history, ECOG performance status, and chemotherapy characteristics. The probability of ethanol-induced symptoms decreased significantly (p < 0.001) by 0.97-fold when age increased by 1 year. When the docetaxel dose was increased by 1 mg, the probability of occurrence of ethanol-induced symptoms increased by statistical significance (p=0.007) by 1.02-fold. When the amount of ethanol in the docetaxel formulation increased by 1 g, the probability of ethanol-induced symptoms increased by 2.38-fold, which was statistically significant (p=0.007). There was no significant difference in the occurrence of side effects between the docetaxel monotherapy and combination therapy groups (Table 4). Due to very low blood alcohol concentration levels, the odds ratio was difficult to estimate. The occurrence of symptoms was compared to the amount of ethanol, divided into categories of < 2, 2–3, and ≥ 3. Most of the symptoms occurred in the 2–3 g of ethanol, which included most subjects (S6 Table). There was no statistically significant difference according to body mass index. According to a more detailed analysis of the occurrence of symptoms within 30 minutes of the completion of docetaxel injection, the docetaxel dose (odds ratio, 1.02; 95% confidence interval, 1.00 to 1.04; p=0.019) and amount of ethanol in the docetaxel formulation (odds ratio, 2.65; 95% confidence interval, 1.17 to 6.00; p=0.019) were significantly associated with the occurrence of ethanol-induced symptoms. Analysis of the occurrence of each ethanol-induced symptom according to sex revealed that the occurrence rate of facial flushing was 5.4% (4/74 patients) in males and 22.6% (85/377 patients) in females, and this difference was statistically significant (p < 0.001). It was also found that the probability of facial flushing increased by 1.02-fold when the docetaxel dose was increased by 1 mg (p=0.026). When the amount of ethanol was increased by 1 g, the probability of facial flushing increased 2.46-fold (p=0.026).
DiscussionDespite the recent development of many anticancer drugs, cytotoxic anticancer drugs are still the basis of chemotherapy, either monotherapy or combination regimens. Most cytotoxic anticancer drugs have lipophilic properties, rendering it difficult to formulate for intravenous injections. Ethanol is a commonly used solvent to increase solubility when manufacturing anticancer drugs for injection. According to a recent study conducted by Hiver et al. [11] on ethanol contained in anticancer drugs available in France, the content of ethanol contained in each anticancer drug was 91 to 400 mg/mL for docetaxel, 395 to 440 mg/mL for gemcitabine, 385 to 402 mg/mL for paclitaxel, and 241 to 248 mg/mL for etoposide, respectively. In addition, the analysis showed that 3,172 of the 63,613 chemotherapy preparations (4.99%) contained more than 3 g of ethanol [11]. Several previous studies have reported that the concentration of ethanol in the blood may increase, and ethanol-induced symptoms may occur after administering anticancer drug-containing ethanol to a patient. It is an important issue that is not an adverse effect of the anticancer drug but an adverse effect of ethanol as a solvent. Aomori et al. conducted a study to measure the ethanol concentration in exhaled gas after the administration of ethanol-containing paclitaxel. The average ethanol concentration in exhaled breath immediately after intravenous infusion of paclitaxel was 0.028±0.015 mg/L (±standard deviation). The estimated blood ethanol concentration was 0.06±0.03 mg/mL. Several patients complained of facial flushing or headaches after the end of intravenous infusion [2]. Webster et al. [5] reported that the average plasma ethanol concentration after administration of paclitaxel was 0.006–0.033 g/dL. Yagi et al. [12] investigated the patient’s tolerability to alcohol-contained paclitaxel based on the aldehyde dehydrogenase 2 (ALDH2) genotype. Ten patients who underwent chemotherapy containing paclitaxel were questioned about 16 alcohol-related symptoms. Five patients (50%) complained of alcohol-related symptoms after paclitaxel infection. Hot flushes and somnolence were the most common alcohol-related symptoms experienced by patients (40% of the patients). All alcohol-related symptoms were grade 1 (Common Terminology Criteria for Adverse Events, ver. 4.0) [12]. Diez-Fernandez et al. [3] investigated the ethanol-induced symptoms in patients receiving ethanol-containing gemcitabine. The mean administered ethanol dose was 15.81±2.25 g. The estimated blood ethanol concentration was 0.033 g/dL according to the Widmark formula. The overall incidence of ethanol-related symptoms was 33.3% in patients treated with ethanol-containing gemcitabine. The most common symptoms were dizziness (29.2%) and unsteady walking (16.7%). Although the difference was not statistically significant, the incidence of ethanol-related symptoms in females was higher [3]. Mirza and Mithal [4] reported a patient who developed alcohol intoxication with a new formulation of docetaxel, which contained a higher quantity of ethanol. The patient complained of blurred vision, drowsiness, imbalance, confusion, diplopia, and dizziness for 3 hours. After the event, the patient received the older formulation again, and the symptoms did not recur [4]. In 2014, based on this case report, the U.S. FDA warned that the intravenous chemotherapy drug docetaxel contains ethanol, also known as alcohol, which may cause patients to experience intoxication or feel drunk during and after treatment. The U.S. FDA also mentioned that healthcare professionals should consider the alcohol content of docetaxel when prescribing or administering the drug to patients, particularly in those whose alcohol intake should be avoided or minimized and when using it in conjunction with other medications. In the present study, the overall occurrence rate of ethanol-induced symptoms related to the administration of ethanol-containing docetaxel was 44.3%. According to the results of previous studies and the present study, it could be suggested that ethanol contained in the docetaxel formulation has adverse effects in addition to its role as a solubilizing agent. In this study, facial flushing was the most common ethanol-induced symptom, which is relatively non-dangerous. Dizziness, one of the symptoms requiring more careful attention because it can be hazardous, occurred in 17.5% of patients. Although infrequent, unsteady walking and impaired balance, which can cause serious problems for patient safety, occurred in 4.2% and 3.3% of patients, respectively. These symptoms are also likely to cause accidents when driving a car or performing dangerous tasks. In the present study, according to the results of the analysis of the time of onset of ethanol-induced symptoms, approximately 20% of patients at each time point developed symptoms during docetaxel injection and immediately after the end of docetaxel injection. In contrast, the occurrence rate of 29% 24 hours after the end of the docetaxel injection suggests that some patients may develop symptoms later. Therefore, it is recommended that physicians and medical staff observe the patient for a more extended period for the possibility of delayed symptoms.
Although the ethanol content varies depending on the manufacturer, when ethanol is administered intravenously rather than from drinking alcohol, a higher blood ethanol concentration can be reached even with a small amount. In addition, intravenous ethanol may have a significant negative impact because of its direct effects on the central nervous system by bypassing first-pass metabolism. Ethanol is metabolized by alcohol dehydrogenase and ALDH2. People with low ALDH2 activity are more sensitive to physical changes caused by alcohol. In particular, Asians have genetically lower ALDH2 activity than Westerners, and ethanol-induced symptoms such as hot flashes, nausea, and tachycardia after drinking are more frequent [13]. In addition to genetically low ALDH2 activity, factors that can affect the expression of ethanol-induced symptoms include sex, ethanol dose, ethanol administration rate, age, body weight, comorbidities, and concomitant medications [14,15]. Therefore, the toxicity caused by ethanol in anticancer drugs varies from mild symptoms to severe ethanol poisoning. In particular, elderly, underweight, or female patients may be more susceptible to ethanol-induced symptoms [16–19]. The present study found that sex, presence of underlying disease, age, docetaxel dose, and docetaxel-containing ethanol amount were significantly associated with the occurrence of ethanol-induced symptoms. The occurrence rate of ethanol-induced symptoms was 32.4% in men and 46.7% in women. The difference by sex was a predictable result due to the quantitative difference in ALDH2 activity between males and females [16,17,19]. Elderly patients are also known to be vulnerable to ethanol toxicity. Changes in ethanol metabolism and the distribution volume of water in elderly patients promote the development of ethanol intoxication [18]. However, in the present study, it was found that the occurrence decreased with increasing age. Although it is difficult to explain the cause of this result, one possible explanation is that elderly patients may not be sensitive to various symptoms. This study demonstrated a significantly higher occurrence of ethanol-induced symptoms in patients without underlying disease than in those with underlying disease. Many medications can interact with alcohol, thereby altering the metabolism or effects of alcohol [20]. Therefore, a possible reason for the significantly lower occurrence of ethanol-induced symptoms in patients with underlying diseases may be the enzymes that metabolize ethanols induced by exposure to various drugs [21], and the underlying diseases may have reduced the pharmacological response to ethanol.
There are some limitations to this study. First, it is impossible to completely distinguish whether the various symptoms collected in this study and classified as ethanol-induced symptoms are caused by docetaxel drugs, ethanol contained in docetaxel formulations, or related to the other drugs before and after docetaxel injections. However, an analysis showed a tendency to increase the occurrence of ethanol-induced symptoms depending on the amount of ethanol administered. Furthermore, comparing the occurrence of ethanol-induced symptoms by classifying the dose range of ethanol administered, we found that ethanol-induced symptoms tended to occur more in patients administered 2–3 g than in patients administered less than 2 g. Therefore, the symptoms collected and classified as ethanol-induced syndrome are likely related to ethanol in the docetaxel formulation. Second, this study was conducted through a competitive registration method without restrictions or adjustments when registering study patients. About 80% of the study patients were breast cancer patients. Therefore, the sex of the study patients was not balanced between men and women, and the proportion of female patients was high. Because the results showed a high occurrence of ethanol-induced symptoms in women, the overall occurrence of ethanol-induced symptoms in this study may have been overestimated.
This study is valuable as the first large-scale, prospective study to present essential data on the frequency, aspects, and factors related to the occurrence of ethanol-induced symptoms after administration of docetaxel-containing ethanol. It was confirmed that the occurrence of ethanol-induced symptoms was not low in patients receiving docetaxel-containing ethanol and that there were more vulnerable patients according to sex, age, and docetaxel dose. Based on the results of this study, it is necessary to provide guidelines for physicians and medical staff prescribing and administering ethanol-containing docetaxel. Physicians need to pay more attention to the occurrence of ethanol-induced symptoms and prescribe ethanol-free or low-ethanol-containing formulations to high-risk patients. In addition, patients receiving ethanol-containing docetaxel should avoid driving a car or engaging in dangerous activity for several hours after the end of docetaxel infusion. Conversely, when manufacturing anticancer drugs, pharmaceutical companies need to reduce the amount of ethanol or develop other solubilizing agents that can replace ethanol or ethanol-free formulations.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The protocol was approved by the Hanyang University Guri Hospital institutional review boards (approval number: GURI 2020-04-020). The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines defined by the International Conference on Harmonization. All study patients provided written informed consent prior to enrollment. Author Contributions Conceived and designed the analysis: Won YW. Collected the data and contributed data analysis: Won YW, Kang JH, Kwon JH, Koo DH, Kang JH, Maeng CH, Ahn HK, Oh SY, Lee DW, Sohn J, Oh SY, Lee KH, Koh SJ, Lee KS, Kim CK, Kim JY, Ji JH, Kim SB, Ha JY, Kim HY. Performed the analysis: Won YW, Kwon JH, Koo DH, Kang JH. Wrote the paper: Won YW. Final approval of manuscript: Won YW, Kwon JH, Koo DH, Kang JH, Maeng CH, Ahn HK, Oh SY, Lee DW, Sohn J, Oh SY, Lee KH, Koh SJ, Lee KS, Kim CK, Kim JY, Ji JH, Kim SB, Ha JY, Kim HY. Fig. 1The occurrence rates for each ethanol-induced symptom. a)Category 1 symptoms: dizziness, difficulty speaking, unsteady walking, impaired balance, mood swings, slower reactions, b)Category 2 symptoms: facial flushing, headache, nausea, palpitation, blurred vision, c)Occurrence rate (%) (95% confidence interval). 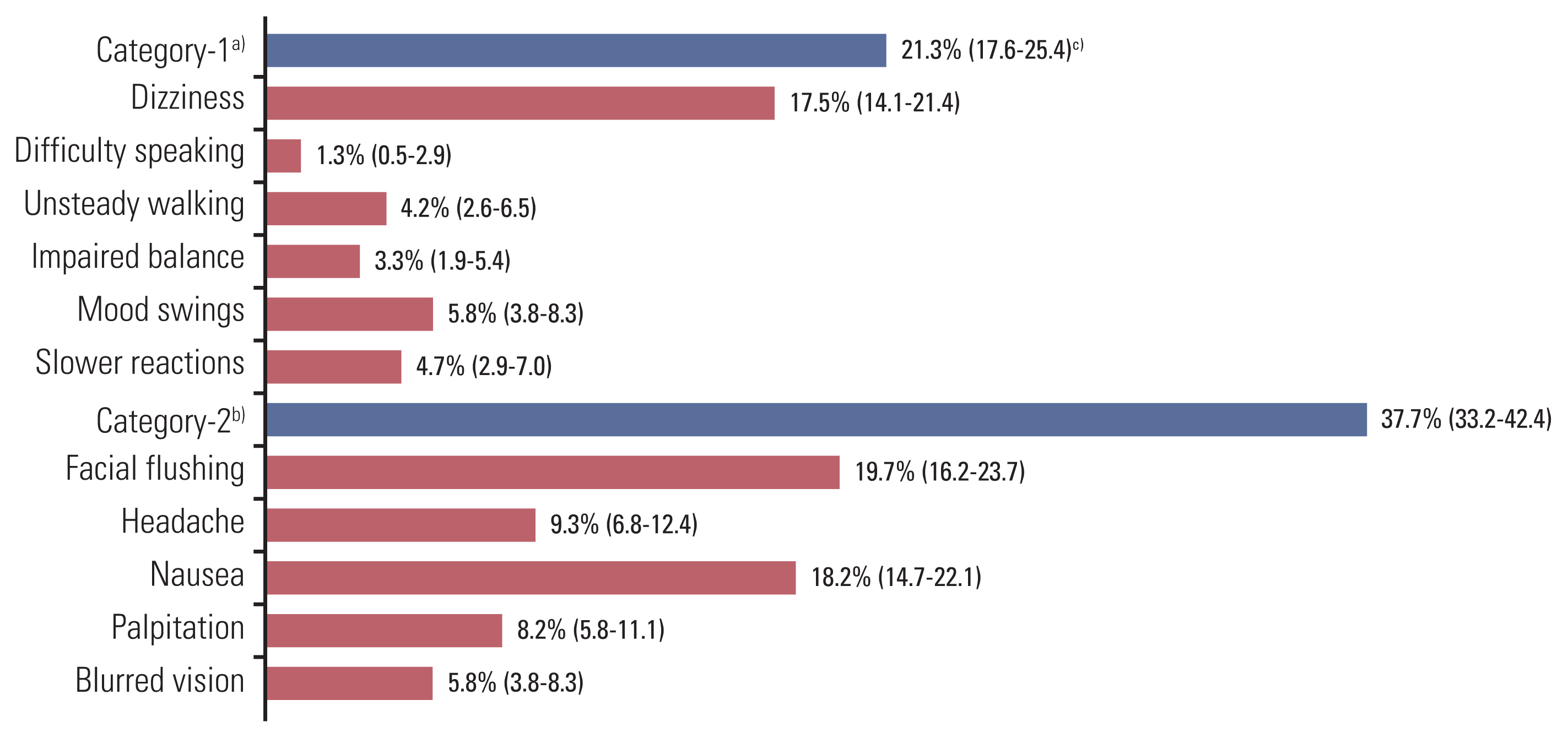
Table 1Baseline characteristics of the study patients Table 2Detailed information of docetaxel administration
Table 3The occurrence rate of ethanol-induced symptoms at each time point Table 4Risk factors for overall ethanol-induced symptom References1. Fries H, Hitzschke M, Lordick F. A different kind of relapse: ethanol as an additive in chemotherapy formulations. Oncol Res Treat. 2019;42:350–3.
2. Aomori T, Makino H, Sekizuka M, Hashita T, Araki T, Iizuka K, et al. Effect of ethanol in paclitaxel injections on the ethanol concentration in exhaled breath. Drugs R D. 2012;12:165–70.
3. Diez-Fernandez R, Vazquez-Sanchez R, Lopez-Esteban L, Enrech-Frances S, Sanchez-Pena AM, Diaz-Paniagua L, et al. Ethanol-induced symptoms in patients receiving gemcitabine diluted from a concentrate for solution for infusion containing ethanol. J Oncol Pharm Pract. 2018;24:511–6.
4. Mirza A, Mithal N. Alcohol intoxication with the new formulation of docetaxel. Clin Oncol (R Coll Radiol). 2011;23:560–1.
5. Webster LK, Crinis NA, Morton CG, Millward MJ. Plasma alcohol concentrations in patients following paclitaxel infusion. Cancer Chemother Pharmacol. 1996;37:499–501.
6. U.S. Food and Drug Administration. FDA Drug Safety Communications: FDA warns that cancer drug docetaxel may cause symptoms of alcohol intoxication after treatment. Silver Spring, MD: U.S. Food and Drug Administration; 2016.
7. Vonghia L, Leggio L, Ferrulli A, Bertini M, Gasbarrini G, Addolorato G, et al. Acute alcohol intoxication. Eur J Intern Med. 2008;19:561–7.
8. Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. Hoboken, NJ: John Wiley & Sons; 2013.
9. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72.
11. Hiver Q, Henry H, Vasseur M, Cuvelier E, Le Rhun E, Turpin A, et al. Ethanol exposure during the intravenous administration of chemotherapeutic drugs: an analysis of clinical practice and a literature review. JCO Oncol Pract. 2022;18:e710–20.
12. Yagi T, Fujiishi K, Hasegawa A, Otsuka T, Yoshinami T, Nishio M, et al. Aldehyde dehydrogenase 2 genotype in tolerability of alcohol contained in paclitaxel in Japanese breast cancer patients. Breast Cancer. 2019;26:229–34.
13. Iwahashi K, Suwaki H. Ethanol metabolism, toxicity and genetic polymorphism. Addict Biol. 1998;3:249–59.
15. Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–57.
16. Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–7.
17. Kwo PY, Ramchandani VA, O’Connor S, Amann D, Carr LG, Sandrasegaran K, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998;115:1552–7.
18. Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care. 2008;11:21–6.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||