AbstractPurposeThe benefit of adjuvant chemotherapy following curative-intent surgery in pancreatic ductal adenocarcinoma (PDAC) patients who had received neoadjuvant FOLFIRINOX is unclear. This study aimed to assess the survival benefit of adjuvant chemotherapy in this patient population.
Materials and MethodsThis retrospective study included 218 patients with localized non-metastatic PDAC who received neoadjuvant FOLFIRINOX and underwent curative-intent surgery (R0 or R1) between January 2017 and December 2020. The association of adjuvant chemotherapy with disease-free survival (DFS) and overall survival (OS) was evaluated in overall patients and in the propensity score matched (PSM) cohort. Subgroup analysis was conducted according to the pathology-proven lymph node status.
ResultsAdjuvant chemotherapy was administered to 149 patients (68.3%). In the overall cohort, the adjuvant chemotherapy group had significantly improved DFS and OS compared to the observation group (DFS: median, 13.8 months [95% confidence interval (CI), 11.0 to 19.1] vs. 8.2 months [95% CI, 6.5 to 12.0]; p < 0.001; and OS: median, 38.0 months [95% CI, 32.2 to not assessable] vs. 25.7 months [95% CI, 18.3 to not assessable]; p=0.005). In the PSM cohort of 57 matched pairs of patients, DFS and OS were better in the adjuvant chemotherapy group than in the observation group (p < 0.001 and p=0.038, respectively). In the multivariate analysis, adjuvant chemotherapy was a significant favorable prognostic factor (vs. observation; DFS: hazard ratio [HR], 0.51 [95% CI, 0.36 to 0.71; p < 0.001]; OS: HR, 0.45 [95% CI, 0.29 to 0.71; p < 0.001]).
IntroductionPancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies with a 5-year survival rate of less than 10%. Only 20% of patients have surgically resectable disease at the time of diagnosis [1–3]. However, even after curative-intent resection, approximately 75% of patients develop recurrence within 2 years, and recurrence occurs more frequently in the absence of adjuvant chemotherapy [1].
For patients who undergo upfront surgery for localized PDAC, adjuvant chemotherapy is the standard of care [4]. Adjuvant modified FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin) and gemcitabine plus capecitabine are the preferred chemotherapy regimens as adjuvant chemotherapy for patients with resected PDAC based on their improved survival outcomes compared to gemcitabine monotherapy in phase 3 trials [5–7].
Recently, neoadjuvant chemotherapy has been widely used for the management of patients with borderline resectable pancreatic cancer (BRPC) and locally advanced pancreatic cancer (LAPC) [8–13]. There is limited evidence to recommend a specific neoadjuvant chemotherapy regimen because of the lack of prospective comparative trials. However, FOLFIRINOX has been widely used based on the better objective response rates and survival outcomes than gemcitabine among patients with metastatic PDAC [14–16]. In meta-analyses of FOLFIRINOX for BRPC and LAPC, conversion surgery could be achieved in 67.8% of BRPC and 25.9% of LAPC patients [14,16].
While the number of patients who undergo surgery following neoadjuvant FOLFIRINOX is increasing, there is only limited data available to guide physicians as to whether adjuvant chemotherapy can improve the survival outcomes in this patient population [17,18]. Therefore, we conducted a retrospective analysis to investigate the survival benefit of adjuvant chemotherapy in patients with resected PDAC after neoadjuvant FOLFIRINOX.
Materials and Methods1. PatientsBetween January 2017 and December 2020, a total of 1,100 patients who underwent surgery for the management of localized non-metastatic pancreatic cancer were identified from the database of Asan Medical Center, Seoul, Korea. Among them, 250 patients received at least 1 cycle of neoadjuvant FOLFIRINOX before surgery. After the exclusion of patients with macroscopic residual disease (R2), histological types other than ductal adenocarcinoma and death within 2 months after surgery, 218 patients with localized PDAC who received neoadjuvant FOLFIRINOX and underwent pancreatectomy were included in this analysis (S1 Fig.). This study was approved by the Institutional Review Board of the Asan Medical Center, Seoul, Korea (IRB approval number: 2021-1282). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
2. Data collection and definitionsPatients’ characteristics including age, sex, and Eastern Cooperative Oncology Group (ECOG) performance status, pathological findings, and data related to treatment and survival were acquired from the electronic medical records. The cancer antigen (CA) 19-9 level was measured at the time of diagnosis, the tumor response evaluation, and pre-/post-operative periods within 1 and 6 weeks from surgery. Baseline status of resectability at diagnosis was classified according to the National Comprehensive Cancer Network (NCCN) criteria [19].
Pathological findings included pathological tumor stage, node stage, resection margin status, lymphovascular invasion or perineural invasion and were graded by the American Joint Committee on Cancer (AJCC), 8th edition [20]. R1 resection was defined as microscopic evidence of a tumor within 1 mm of the resection margin. Pathologic response was graded according to the College of American Pathologists (CAP) guideline [21].
FOLFIRINOX consisted of oxaliplatin (85 mg/m2 body surface area), irinotecan (150 or 180 mg/m2), leucovorin (400 mg/m2), and fluorouracil (2,400 mg/m2 over 46 hours) with or without a bolus of fluorouracil (400 mg/m2), every 2 weeks, as described previously [5,15]. Adjuvant chemotherapy was defined as at least 1 cycle of postoperative systemic chemotherapy. Postoperative chemoradiation was recommended for R1 resected patients, and patients who received only postoperative chemoradiation were not considered to have received adjuvant chemotherapy. Disease-free survival (DFS) was defined as the interval between surgery and recurrence or death from any etiology, whichever occurred first, and overall survival (OS) was the interval between surgery and death from any etiology.
3. Statistical analysisCategorical variables are presented as frequencies and proportions and continuous variables are presented as medians with interquartile ranges (IQRs). Survival was assessed using Kaplan-Meier survival curves and presented as median DFS and OS with corresponding 95% confidence intervals (CIs). Cox proportional hazards models were used for univariate and multivariate analyses and the outcomes are presented as the hazard ratio (HR) and 95% CI. The variables with p values < 0.2 in univariate analysis were included in the multivariate analysis.
Propensity score matching (PSM) was conducted to create balanced cohorts, including variables of age, sex, ECOG performance score, tumor extent at the time of diagnosis, pathological T category, N category, resection margin status, number of cycles of neoadjuvant FOLFIRINOX and preoperative CA 19-9 level. Patients were matched based on the propensity scores using the 1:1 nearest-neighbor method. Standardized mean difference was adopted with a value < 0.1 indicating a good balance. All analyses were performed using R statistical software ver. 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results1. Patient characteristicsAmong 218 patients with PDAC who underwent curative-intent surgery following neoadjuvant FOLFIRINOX, adjuvant chemotherapy was administered to 149 patients (68.3%). The patients’ baseline characteristics are summarized in Table 1. The number of cycles of neoadjuvant FOLFIRINOX was significantly higher in the observation group compared to the adjuvant chemotherapy group (median [IQR], 9 [7–10] vs. 7 [5–8]; p < 0.001). At the time of diagnosis, patients were classified into resectable pancreatic cancer (n=7, 10.1% and n=15, 10.1%), BRPC (n=39, 56.5% and n=103, 69.6%), and LAPC (n=23, 33.3% and n=30, 20.3%) according to the NCCN criteria in the observation group and the adjuvant chemotherapy group, respectively, and there was no statistical difference (p=0.105). There was no significant difference in any other characteristics, including the tumor location, surgical types, resection margin status, pathologic stage, or tumor response to FOLFIRINOX between the two groups.
In the adjuvant chemotherapy group, FOLFIRINOX (n=98, 65.8%) was administered most frequently followed by gemcitabine monotherapy (n=39, 26.2%) and gemcitabine-capecitabine (n=4, 2.7%). The median duration of adjuvant chemotherapy was 2.5 months (95% CI, 2.3 to 3.0) in the adjuvant chemotherapy group. In the observation group, adjuvant chemotherapy was not administered because of the following reasons: sufficient preoperative exposure to FOLFIRINOX based on the number of cycles and pathologic response as determined by physicians (at least 8 cycles of preoperative FOLFIRINOX or near complete response (CAP grade 1) were considered as sufficient exposure although there are no globally established criteria yet) (n=23), medically not fit for adjuvant chemotherapy by a shared decision made by physicians and patients (n=35), patient’s wishes (n=6), and postoperative complications (n=3). Postoperative chemoradiotherapy was done in seven (10.1%) patients of the observation group and 11 (7.4%) patients of the adjuvant chemotherapy group (p=0.480).
2. Survival outcomes of the overall cohortWith a median follow-up duration of 26.6 months (95% CI, 24.8 to 29.9), patients in the adjuvant chemotherapy group showed significantly better survival outcomes compared to those in the observation group, with a median DFS of 13.8 months (95% CI, 11.0 to 19.1) vs. 8.2 months (95% CI, 6.5 to 12.0), respectively (p < 0.001); and a median OS of 38.0 months (95% CI, 32.2 to not assessable [NA]) vs. 25.7 months (95% CI, 18.3 to NA), respectively (p=0.005) (Fig. 1A and B).
In the subgroup analysis according to the lymph node status, adjuvant chemotherapy was significantly associated with a better DFS and OS than observation in patients with negative lymph nodes (ypN0; p=0.002 and p=0.020, respectively) (Fig. 1C and D), and showed a marginal relationship for better DFS and OS in those with positive lymph nodes (ypN+; p=0.058 and p=0.058, respectively) (Fig. 1E and F).
In the adjuvant chemotherapy group, there was no significant difference in the survival outcomes between the patients with adjuvant gemcitabine-based chemotherapy and FOLFIRINOX with a median DFS of 10.7 months (95% CI, 8.8 to 20.6) vs. 17.2 months (95% CI, 12.0 to 23.1) (p=0.565); and a median OS of 35.0 months (95% CI, 30.1 to NA) vs. 38.0 months (95% CI, 32.7 to NA) (p=0.393) (S3 Fig.). Among the patients who received adjuvant FOLFIRINOX, there was no significant difference in survival outcomes between patients who received less than 12 cycles of perioperative FOLFIRINOX and those who received more than 12 cycles (< 12 cycles [n=43] vs. ≥ 12 cycles [n=54]; DFS: median, 14.6 months [95% CI, 10.5 to NA] vs. 17.4 months [95% CI, 10.9 to NA]; p=0.393; OS: median, not reached [95% CI, 38.0 to NA] vs. 36.6 months [95% CI, 29.5 to NA]; p=0.992) (S4 Fig.).
In the observation group, there was no statistically significant difference in survival outcomes according to the reasons for not receiving adjuvant chemotherapy, however, patients with medically unfit conditions showed a tendency for the worst prognosis. (sufficient preoperative exposure to FOLFIRINOX vs. medically unfit conditions and patient’s wishes vs. postoperative complications; DFS: median, 13.2 months [95% CI, 10.2 to 22.0] vs. 4.7 months [95% CI, 3.8 to 10.3] vs. 19.7 months [95% CI, 19.4 to NA]; p=0.257; OS: median, not reached [95% CI, 23.5 to NA] vs. 19.6 months [95% CI, 14.8 to NA] vs. 31.0 months [95% CI, 31.0 to NA]; p=0.193) (S5 Fig.).
The results of the univariate and multivariate analyses for DFS and OS are summarized in Tables 2 and 3. In the multivariate analysis, adjuvant chemotherapy was significantly associated with better DFS (HR, 0.51 [95% CI, 0.36 to 0.71]; p < 0.001) and OS (HR, 0.45 [95% CI, 0.29 to 0.71]; p < 0.001). In addition, elevated preoperative CA 19-9 levels were significantly associated with worse DFS (HR, 1.93 [95% CI, 1.36 to 2.73]; p < 0.001) and OS (HR, 2.05 [95% CI, 1.32 to 3.17]; p=0.001). The presence of lymphovascular invasion was significantly associated with a worse DFS (HR, 1.55 [95% CI, 1.09 to 2.21]; p=0.016) and a positive resection margin was associated with a worse OS (HR, 1.73 [95% CI, 1.00 to 2.97]; p=0.048).
3. Survival outcomes of the PSM cohortIn the PSM cohort, DFS and OS were significantly better in the adjuvant chemotherapy group than in the observation group; a median DFS of 13.8 months (95% CI, 10.7 to 23.1) vs. 7.8 months (95% CI, 4.7 to 11.5), respectively (HR, 0.51 [95% CI, 0.33 to 0.79]; p=0.003) and a median OS of 31.5 months (95% CI, 26.1 to NA) vs. 23.5 months (95% CI, 17.4 to NA), respectively (HR, 0.57 [95% CI, 0.33 to 0.98]; p=0.040) (Fig. 2A and B).
In the subgroup analysis according to the lymph node status, the median DFS and OS were statistically better in the adjuvant chemotherapy group than in the observation group for patients with positive lymph nodes (ypN+; p=0.027 and p=0.049, respectively) (Fig. 2C and D). For patients with negative lymph nodes, the adjuvant chemotherapy group showed significantly better DFS and a tendency for a better OS compared to the observation group (ypN0; p=0.024 and p=0.160, respectively) (Fig. 2E and F).
DiscussionThis retrospective study assessed the clinical implications of adjuvant chemotherapy among 218 patients who underwent curative-intent surgery after neoadjuvant FOLFIRINOX for localized PDAC. In the current study, patients who received adjuvant chemotherapy showed significantly better DFS (median, 13.8 months vs. 8.2 months; p < 0.001) and OS (median, 38.0 months vs. 25.7 months; p=0.005) compared with those who did not. Its benefit remained significant after PSM (DFS: median, 13.8 months vs. 7.8 months; p < 0.001; OS: median, 31.5 months vs. 23.5 months; p=0.038). Furthermore, the trends for the benefit of adjuvant chemotherapy in terms of DFS and OS were consistent regardless of the status of the lymph nodes.
There are no high-level data supporting the use of adjuvant chemotherapy among patients who underwent pancreatectomy after neoadjuvant chemotherapy. Several retrospective analyses have yielded conflicting results, particularly in the subgroup that benefited from adjuvant chemotherapy [17,18,22–25]. Some studies have suggested that postoperative chemotherapy was significantly associated with improved survival for patients with a lower lymph node burden [22,23], whereas one study reported a survival benefit for patients with lymph node positive disease [24]. These early studies, however, included various drugs as neoadjuvant chemotherapy, and a large proportion of patients received only chemoradiation as preoperative treatment [22–24]. A retrospective cohort study using the US National Cancer database showed that adjuvant chemotherapy was associated with a longer survival only in patients with a lymph node ratio (LNR) between 0.01 and 0.149, not in node-negative patients or those with a LNR greater than 0.15 [25], and a recent nationwide retrospective study by Kamarajah et al. [17] found that adjuvant chemotherapy was associated with a survival benefit in patients with ypN0 and ypN1, but not ypN2. These studies are also limited because of the inclusion of various neoadjuvant chemotherapeutic regimens and treatment modalities. Considering that the efficacy of modern-era multiagent chemotherapy regimens such as FOLFIRINOX or gemcitabine plus nab-paclitaxel has been much improved compared to old-fashioned treatments for patients with PDAC, the study population should be homogeneous in terms of neoadjuvant therapy to avoid potential bias.
Our study was based on a homogeneous study population as we included only patients who underwent neoadjuvant FOLFIRINOX followed by surgical resection. The only other study that has assessed the role of adjuvant chemotherapy following FOLFIRINOX and surgery was recently published by the European-African Hepato-Pancreato-Biliary Association [18]. This cohort study included 520 patients who underwent surgical resection after neoadjuvant FOLFIRINOX and showed that adjuvant chemotherapy was associated with improved survival only in the lymph node positive subgroup [18]. In contrast, our findings showed that adjuvant chemotherapy was associated with better survival outcomes compared to observation in the overall patient population and its benefit seems to include both lymph node positive and negative groups. As there were differences in the use of adjuvant chemotherapy (FOLFIRINOX 65.8% in the current study vs. 19.8% in the prior international study and gemcitabine-based chemotherapy [28.9% vs. 58.6%]), this might be a potential reason for discrepancies in the outcomes between these studies.
There is no established data about the regimen or the duration (cycles) of adjuvant chemotherapy following neoadjuvant FOLFIRINOX based on the heterogeneity of neoadjuvant and adjuvant chemotherapeutic regimens and cycles of previous studies. Despite a trend toward better survival for adjuvant FOLFIRINOX in this study (gemcitabine-based vs. FOLFIRINOX; median DFS, 10.7 months vs. 17.2 months, p=0.565; median OS, 35.0 months vs. 38.0 months, p=0.393), it was not statistically significant and still difficult to suggest a specific regimen based on this data due to the small number of samples in each group. In addition, recent data have shown that sequential treatment with two different chemotherapy regimens may be more effective for patients with metastatic pancreatic cancer [26,27]. It may suggest that changing the regimen of adjuvant chemotherapy may be the one of potential therapeutic strategies for certain subgroup of patients (e.g., clinically or pathologically insufficient responsive groups). In terms of the implications of duration or number of cycles of adjuvant chemotherapy, it is also difficult to draw conclusive recommendations based solely on the current analysis due to the limitations of its retrospective nature. Further prospective investigations are warranted to define the optimal regimens or number of cycles of postoperative chemotherapy in patients with resected PDAC after neoadjuvant FOLFIRINOX.
In the multivariate analysis, adjuvant chemotherapy remained significant for better DFS and OS. In addition, an elevated preoperative CA 19-9 level was significantly correlated with a worse DFS and OS, which was consistent with the results of previous studies [28–31]. Although the number of chemotherapy cycles, tumor size, or pathologic response to neoadjuvant therapy have been suggested as prognostic factors in previous studies [28,30,32], these were not significantly associated with DFS and OS in the current study.
Our study has limitations. This was a non-randomized, retrospective study, susceptible to bias. Although PSM was applied to minimize the selection biases, this may not totally exclude the potential biases. Furthermore, patients who could not recover from surgery may be included in the observation group and the inferior survival outcomes in the observation group might be attributable to the undertreatment caused by poor general condition following surgery. In addition, as the median number of cycles of neoadjuvant FOLFIRINOX was significantly higher in the observation group than the adjuvant chemotherapy group, the patients in the adjuvant chemotherapy group might have favorable tumor biology for earlier conversion to the surgery compared to those in the observation group, and this might impact on the clinical outcomes between the two groups.
In PDAC patients who underwent surgery following neoadjuvant FOLFIRINOX, adjuvant chemotherapy may be associated with improved survival outcomes. Its benefit is not affected by the lymph node status. These findings suggest that adjuvant chemotherapy could be considered in all patients who have completed neoadjuvant chemotherapy and curative-intent surgery, whenever patients are medically fit for chemotherapy. Prospective, large-scale, multinational, multicenter randomized studies are necessary to confirm our findings.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website https://www.e-crt.org.
NotesEthical Statement This study was approved by the Institutional Review Board of the Asan Medical Center, Seoul, Korea (IRB approval number: 2021-1282). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Author Contributions Conceived and designed the analysis: Yoo C, Kim SC. Collected the data: Lee SH, Hwang DW, Yoo C, Kim KP, Kang S, Jeong JH, Oh D, Song TJ, Lee SS, Park DH, Seo DW, Park JH, Song KB, Lee JH, Lee W, Park Y, Kwak BJ, Chang HM, Ryoo BY, Kim SC. Contributed data or analysis tools: Lee SH, Hwang DW, Yoo C, Kim KP, Kang S, Jeong JH, Oh D, Song TJ, Lee SS, Park DH, Seo DW, Park JH, Song KB, Lee JH, Lee W, Park Y, Kwak BJ, Chang HM, Ryoo BY, Kim SC. Performed the analysis: Lee SH, Hwang DW, Yoo C. Wrote the paper: Lee SH, Yoo C. AcknowledgmentsThis work was supported in part by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea [grant numbers 2021IT-0008]. The funder had no role in the design, analysis, or interpretation of the study.
Fig. 1Survival outcomes according to adjuvant chemotherapy in the overall (unmatched) cohort. (A) Disease-free survival in overall patients. (B) Overall survival in overall patients. (C) Disease-free survival in patients with positive lymph nodes. (D) Overall survival in patients with positive lymph nodes. (E) Disease-free survival in patients with negative lymph nodes. (F) Overall survival in patients with negative lymph nodes. CI, confidence interval; NA, not assessable. 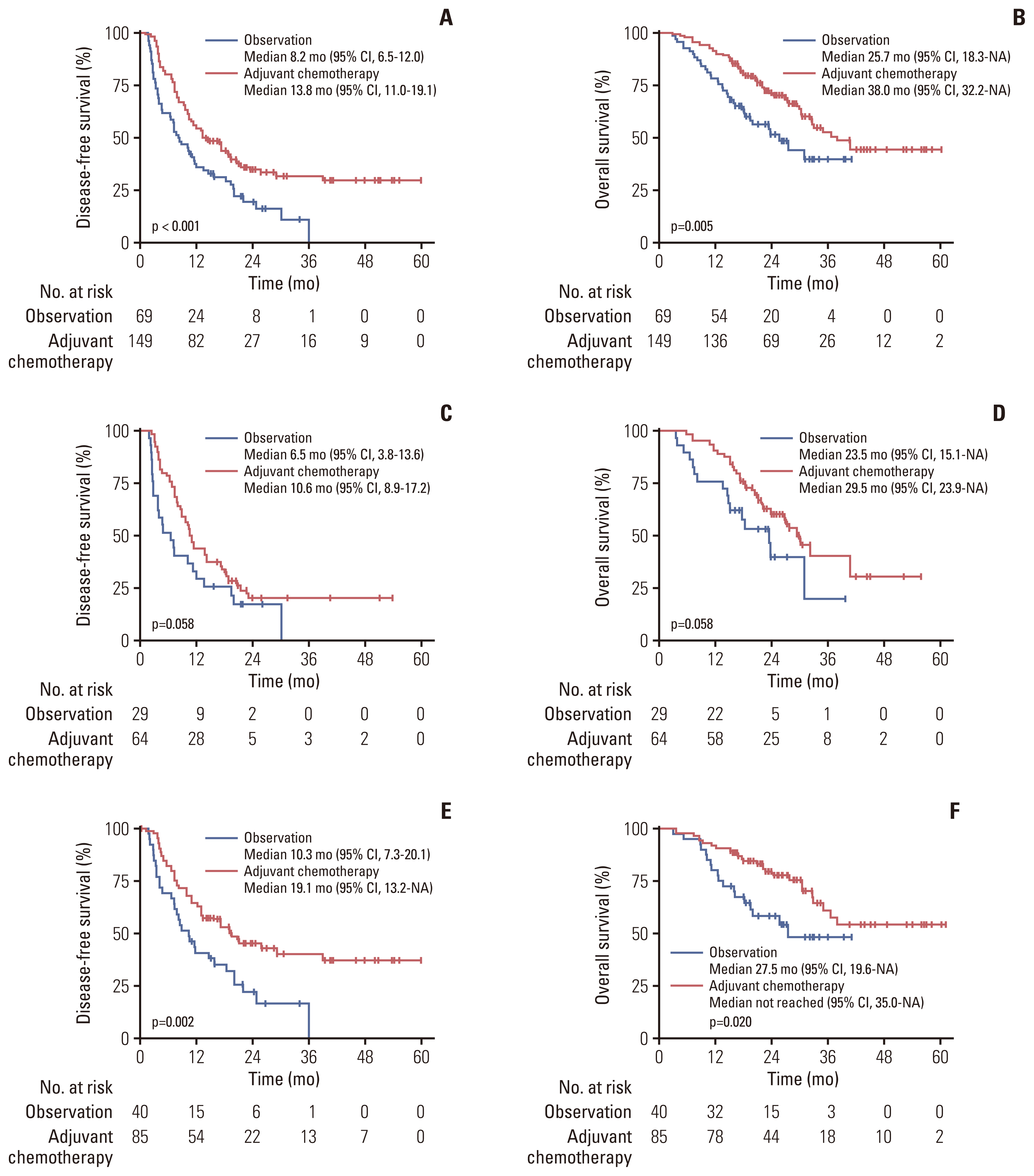
Fig. 2Survival outcomes according to adjuvant chemotherapy in the matched cohort. (A) Disease-free survival in overall patients. (B) Overall survival in overall patients. (C) Disease-free survival in patients with positive lymph nodes. (D) Overall survival in patients with positive lymph nodes. (E) Disease-free survival in patients with negative lymph nodes. (F) Overall survival in patients with negative lymph nodes. CI, confidence interval; NA, not assessable. 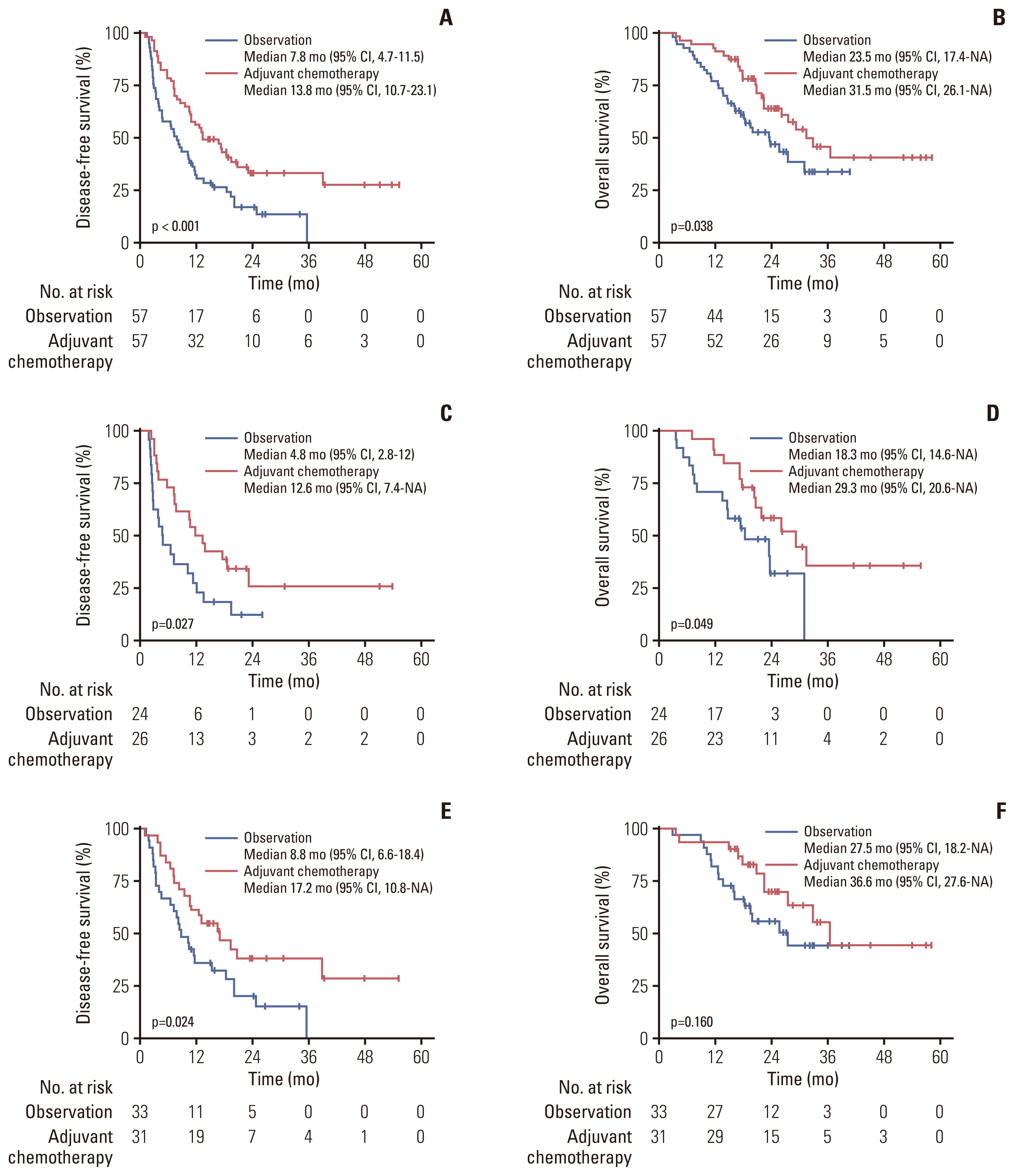
Table 1Baseline characteristics in unmatched and matched cohorts
Values are presented as number (%) unless otherwise indicated. BRPC, borderline resectable pancreatic cancer; CA 19-9, cancer antigen 19-9; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance score; IQR, interquartile range; LAPC, locally advanced pancreatic cancer; PD, pancreaticoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy; UNL, upper normal limit; WNL, within normal limits. Table 2Univariate and multivariate analysis for disease-free survival BRPC, borderline resectable pancreatic cancer; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; FOLFIRINOX, fluorouracil, leucovorin, irinotecan and oxaliplatin; HR, hazard ratio; LAPC, locally advanced pancreatic cancer; PD, pancreaticoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy. Table 3Univariate and multivariate analysis for overall survival BRPC, borderline resectable pancreatic cancer; CA 19-9, cancer antigen 19-9; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance score; FOLFIRINOX, fluorouracil, leucovorin, irinotecan and oxaliplatin; HR, hazard ratio; LAPC, locally advanced pancreatic cancer; PD, pancreaticoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy. References1. Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267:936–45.
2. Maeda S, Unno M, Yu J. Adjuvant and neoadjuvant therapy for pancreatic cancer. J Pancreatol. 2019;2:100–6.
3. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61.
4. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–68.
5. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–406.
6. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
7. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81.
8. Nagakawa Y, Sahara Y, Hosokawa Y, Murakami Y, Yamaue H, Satoi S, et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann Surg Oncol. 2019;26:1629–36.
9. Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10:245.
10. Unno M, Hata T, Motoi F. Long-term outcome following neoadjuvant therapy for resectable and borderline resectable pancreatic cancer compared to upfront surgery: a meta-analysis of comparative studies by intention-to-treat analysis. Surg Today. 2019;49:295–9.
11. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105:946–58.
12. Yoo C, Shin SH, Kim KP, Jeong JH, Chang HM, Kang JH, et al. Clinical outcomes of conversion surgery after neoadjuvant chemotherapy in patients with borderline resectable and locally advanced unresectable pancreatic cancer: a single-center, retrospective analysis. Cancers (Basel). 2019;11:278.
13. Yoo C, Lee SS, Song KB, Jeong JH, Hyung J, Park DH, et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer. 2020;123:362–8.
14. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–10.
15. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
16. Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. 2019;111:782–94.
17. Kamarajah SK, White SA, Naffouje SA, Salti GI, Dahdaleh F. Adjuvant chemotherapy associated with survival benefit following neoadjuvant chemotherapy and pancreatectomy for pancreatic ductal adenocarcinoma: a population-based cohort study. Ann Surg Oncol. 2021;28:6790–802.
18. van Roessel S, van Veldhuisen E, Klompmaker S, Janssen QP, Abu Hilal M, Alseidi A, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6:1733–40.
19. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–61.
20. Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265:185–91.
21. Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas [Internet]. Northfield, IL: College of American Pathologists; c2012. [cited 2022 16 May]. Available from: https://cap.objects.frb.io/protocols/cp-pancreas-exocrine-2016-v3301.pdf
22. Roland CL, Katz MH, Tzeng CW, Lin H, Varadhachary GR, Shroff R, et al. The addition of postoperative chemotherapy is associated with improved survival in patients with pancreatic cancer treated with preoperative therapy. Ann Surg Oncol. 2015;22(Suppl 3):S1221–8.
23. Olecki EJ, Stahl KA, Torres MB, Peng JS, Dixon M, Shen C, et al. Adjuvant chemotherapy after neoadjuvant chemotherapy for pancreatic cancer is associated with improved survival for patients with low-risk pathology. Ann Surg Oncol. 2021;28:3111–22.
24. Barnes CA, Krepline AN, Aldakkak M, Clarke CN, Christians KK, Khan AH, et al. Is adjuvant therapy necessary for all patients with localized pancreatic cancer who have received neoadjuvant therapy? J Gastrointest Surg. 2017;21:1793–803.
25. Swords DS, Francis SR, Lloyd S, Garrido-Laguna I, Mulvihill SJ, Gruhl JD, et al. Lymph node ratio in pancreatic adenocarcinoma after preoperative chemotherapy vs. preoperative chemoradiation and its utility in decisions about postoperative chemotherapy. J Gastrointest Surg. 2019;23:1401–13.
26. Carrato A, Pazo-Cid R, Macarulla T, Gallego J, Jiménez-Fonseca P, Rivera F, et al. Sequential nab-paclitaxel/gemcitabine followed by modified FOLFOX for first-line metastatic pancreatic cancer: The SEQUENCE trial. J Clil Oncol. 2022;40:4022.
27. Rinaldi Y, Pointet AL, Khemissa Akouz F, Le Malicot K, Wahiba B, Louafi S, et al. Gemcitabine plus nab-paclitaxel until progression or alternating with FOLFIRI.3, as first-line treatment for patients with metastatic pancreatic adenocarcinoma: the Federation Francophone de Cancerologie Digestive-PRODIGE 37 randomised phase II study (FIRGEMAX). Eur J Cancer. 2020;136:25–34.
28. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273:341–9.
29. Garnier J, Robin F, Ewald J, Marchese U, Bergeat D, Boudjema K, et al. Pancreatectomy with vascular resection after neoadjuvant FOLFIRINOX: who survives more than a year after surgery? Ann Surg Oncol. 2021;28:4625–34.
30. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients eith borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019;269:733–40.
31. Yoo C, Hwang I, Song TJ, Lee SS, Jeong JH, Park DH, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol. 2020;12:1758835920953294.
32. Pietrasz D, Marthey L, Wagner M, Blanc JF, Laurent C, Turrini O, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015;22(Suppl 3):S1196–205.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||