AbstractPurposeThis study aimed to explore the impact of ABL1–tyrosine kinase inhibitors (TKIs) adherence on the survival of chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) children and clarify the potential predictors of patients’ prognosis from TKIs intake practices.
Materials and MethodsNinety newly diagnosed Ph+ ALL patients who received TKIs were enrolled. We collected the baseline characteristics and adverse events in all children; moreover, TKIs adherence was measured by an eight-item Morisky medication adherence scale (MMAS-8). Progression-free survival (PFS) and overall survival (OS) analysis were performed, and risk factors for PFS and OS were evaluated.
ResultsAmong all patients, 69 cases were regarded as adherers, while 21 were non-adherers. The median duration of TKIs interruption was significantly prolonged in the non-adherence group than in the adherence group (13 [0–101] vs. 56 [11–128], p < 0.001). Additionally, dose reduction occurred in 55.2% of non-adherers versus 23.0% of adherers (p=0.002). The PFS and OS in adherers were significantly higher versus non-adherers (p=0.020 and p=0.039). MMAS-8 score was an independent risk factor for PFS (p=0.010) and OS (p=0.031). Among non-adherers, the median OS was only 23.1% (4.2%–42%) in patients aged ≤ 10 years versus 54.4% (38.8%–70%) in adolescents. Most of the patients who experienced TKIs non-adherence suffered pancytopenia.
ConclusionTKIs adherence during treatment significantly influenced the survival of pediatric Ph+ ALL patients, and non-adherers with age ≤ 10 years were more vulnerable to TKIs disruption. The cumulative TKIs dose should be especially emphasized to patients with age ≤ 10 years, which may result in an inferior achievement of relevant treatment milestones.
IntroductionAcute lymphoblastic leukemia (ALL) is regarded as the most common cancer in children, and contemporary studies of childhood ALL have shown improved 5-year overall survival (OS) rates exceeding 90% [1]. Philadelphia chromosome–positive (Ph+) status or BCR-ABL1 positive presents in 2%–5% of pediatric ALL cases, and it impacts the prognosis and treatment stratification in childhood ALL. A study from the St Jude Children’s Research Hospital indicated that 15 Ph+ ALL children treated with dasatinib had an excellent 5-year progression-free survival (PFS) rate of 71%, despite hematopoietic stem cell transplantation (HSCT) limited to only one patient [2]. Recently, a study from the Chinese Children’s Cancer Group compared the efficacy of imatinib with that of dasatinib in children with Ph+ ALL. By the study design, only 2% of the patients with minimal residual disease (MRD) ≥ 1% after remission induction received HSCT [3]. These findings suggest that HSCT is unnecessary until pediatric Ph+ ALL patients suffering from relapsed or refractory ALL, and tyrosine kinase inhibitors (TKIs) on top of chemotherapy were still the first choice by achieving an optimal therapeutic level. Thus, concomitant use of BCR-ABL1 TKIs, such as dasatinib, ponatinib, and olverembatinib in Ph+ ALL treatment has improved the prognosis of these patients [4–6].
However, adhering to the BCR-ABL1 TKI regimen is an essential issue for patients with Ph+ ALL. A prolonged maintenance phase with daily self-administered TKIs has been required in durable first clinical remissions in Ph+ ALL patients. Adherence to the regimen is a process by which patients take their TKIs as prescribed and mainly includes 3 phases: initiation, implementation, and discontinuation [7]. Until now, it was still unknown whether low or varying TKIs exposure in the implementation phase, which patients have initiated but have not yet discontinued, may impact the patient’s prognosis. Thus, the objective of this study was to explore the impact of adherence to TKIs on the prognosis of Ph+ ALL children and clarify the potential predictors of patients’ prognosis from TKIs intake practices.
Materials and Methods1. Study design and participant recruitmentThe source population for this study included 90 children with Ph+ ALL at the Division of Pediatric Blood Diseases Center, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College between January 2015 and January 2022. We included Ph+ ALL patients who were (1) younger than 16 years old; (2) in their first clinical remission; (3) received BCR-ABL1 TKIs for more than three months until disease progression or unacceptable toxicity occurs; (4) having a caregiver or designated parents willing to participate in a supervised medication administration and willing to be followed-up. Patients with Down syndrome were excluded, and poor adherence to mercaptopurine or other drugs was also excluded to avoid other reasons affecting the results. The patients were treated according to the Chinese Children’s Cancer Group study ALL-2015 (CCCG-ALL-2015) or CCCG-2020 ALL protocols. The CCCG-ALL-2015 and CCCG-2020 are prospective, multi-institutional clinical trials involving 20 major hospitals and medical centers [4,8]. These protocols were trials for the therapy of childhood ALL, and the treatment schedule was the same for Ph+ ALL in both protocols. In general, prophase treatment with dexamethasone at 6 mg/m2 per day was given for 4 days. Subsequent remission induction consisted of prednisone (dexamethasone), daunorubicin, vincristine, and pegaspargase (days 5–28), followed by cyclophosphamide, cytarabine, and mercaptopurine (days 29–35). BCR-ABL1 TKIs (imatinib [300 mg/m2 per day] or dasatinib [80 mg/m2 per day]) were started after initiation of prophase treatment and continued until the end of therapy for patients with Ph+ ALL. Once fever occurs and infection cannot be ruled out, all chemotherapy and TKI should be suspended. If there is severe hematological toxicity (absolute neutrophil count [ANC] < 0.1×109/L or platelets < 20×109/L), all chemotherapy, including TKIs should be stopped. Persistent ANC < 0.5×109/L or platelets < 100×109/L without improvement by halved myelosuppressive agents (excluding 6-mercaptopurine), the TKI should be reduced by 20%. As a previous study indicated, a new generation of TKIs will be applied if the patients suffer from relapse or disease progression [9].
The data collected included age at diagnosis, sex, parental education level, family income, forms of BCR/ABL, MRD level, the concurrence of IKZF1 deletion, and chromosome karyotypes. TKIs intake practices, such as current TKI type, days of TKI interruption, and TKI dose reduction, were also documented.
2. Adherence assessments and side effects evaluationPrescribed medications involving any changes in type or dose of TKIs by the treating physicians were cross-examined with the protocols and the results of the blood sample (including complete blood count, hepatic function, and coagulation factors II-VII-X). We assessed patient-reported side effects and medication adherence using questionnaires to collect data. The first part of the questionnaire was designed to manage the social characteristics of caregivers, including educational level, annual household income and structure. The second part was a licensed, validated 8-item questionnaire-Morisky medication adherence scale (MMAS-8) to assess the adherence of included patients [10]. According to a previous study, the MMAS-8 score of < 6 and ≥ 6 is regarded as low and high adherence, respectively [11–13]. Our study defined the MMAS-8 score of < 6 as non-adherence. In addition, the third part was to collect the profiles of non-hematologic side effects, such as skin rash, gastrointestinal bleeding, pancytopenia, headache, bone pain, and pleural effusion when taking TKIs. Moreover, altered medication adherence owing to side effects and lack of information about treatment was also documented.
At our institution, patients must identify the primary caregiver (typically their parents or guardians) who will help them during treatment. For this study, we sought participation from the primary caregiver. The survey and adherence evaluation were conducted on days 180 before re-induction therapy via a questionnaire by caregivers. The caregivers of patients who suffered from disease progression or death completed the adherence evaluation right after the events.
The study design and methods complied with the Declaration of Helsinki and were approved by the ethics committee of the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (IRB number: IIT2020020-EC-1). Informed consent was obtained from all subjects.
3. Statistical analysisComparisons of baseline characteristics, side effects, and answers to the MMAS-8 questionnaire regarding adherence were conducted. Statistically significant differences between the two groups were tested using the Mann-Whitney U-test for continuous variables and Pearson chi-squared test for categorical variables. The Spearman correlation analysis was executed to test whether two groups, according to the MMAS-8, scored significantly different in discontinuation of TKIs use. The major cytogenetic response (MCyR) was assessed as the response to therapy six months after treatment. At the same time, the PFS and OS were evaluated from the first day of administration of TKIs until any relapse or death. The Kaplan-Meier method was used to examine the predictors of the PFS and OS by using the log-rank test. A multivariable backward stepwise Cox regression approach was used to examine the relationship between potential risk factors and survival rates in these patients. Hazards ratios (HRs) and 95% confidence intervals (CIs) were presented for all covariates included in the Cox regression model. Two-tailed p=0.05 was set as the statistical significance level. All statistical analysis was performed using GraphPad Prism 8.00 (GraphPad Software Inc., San Diego, CA) and SPSS ver. 21.0 (IBM Corp., Armonk, NY).
Results1. Patient baseline characteristicsAll 90 Ph+ ALL patients who received TKIs during our recruitment period were enrolled. Among all included patients, the median follow-up was 33 months (range, 1 to 85 months). The median age of included patients was 9 years (range, 2 to 17 years), and 57 (63.3%) were male. Adherence assessments were performed for all enrolled participants, which found that 69 cases were regarded as adherers while 21 were non-adherers. Table 1 illustrated the sociodemographic and clinical features of included patients. Moreover, TKIs intake practices were also documented. The patients in the two groups were comparable concerning sociodemographic and clinical characteristics of all patients. TKIs interruption during treatment was reported in 100% of patients in the non-adherence group, which was significantly higher than 78.3% (54/69) in the adherence group (p=0.019). Moreover, the median duration of TKIs interruption was also significantly prolonged in the non-adherence group than in the adherence group (13 [0–101] vs. 56 [11–128], p < 0.001). Additionally, dose reduction occurred in 55.2% (16/29) of non-adherers when compared with 23.0% (14/61) of adherers (p=0.002). In this cohort, the most common causes for treatment interruptions or dose reductions were intolerance of TKIs.
2. Response rate and survival outcomes by TKIs adherenceTo test whether the two groups, according to the MMAS-8, scored significantly different in attitudes toward TKIs use, we performed a Spearman correlation analysis (Fig. 1) [11,13,14]. The results revealed that patients with improved compliance obtained higher scores in a positive attitude to drugs (r=−0.488, p < 0.001).
There was no statistically significant difference in the rates of MCyR between patients in the adherence and non-adherence groups (11.5% vs. 17.2% p=0.452) by six months after treatment. When it comes to the survival outcomes, the PFS for patients in the adherence group and non-adherence group was 73.9% (95% CI, 65 to 82.8) and 33.0% (95% CI, 21.4 to 44.6) (p=0.020) (Fig. 2A). In addition, there was also significantly higher OS in the adherence group in comparison to the non-adherence group (82.2% [95% CI, 74.0 to 90.4] vs. 39.0% [95% CI, 24.5 to 53.5], p=0.039) (Fig. 2B). Among all included patients, 56.7% (51/90) of them have completed the treatment, and the PFS and OS of adherers were also significantly higher than non-adherers (69.7% [95% CI, 59.9 to 79.5] vs. 40.0% [95% CI, 28.9 to 51.1], p=0.032) and (77.8% [95% CI, 68.7 to 86.9] vs. 44.6% [95% CI, 32.8 to 56.4], p=0.049) (Fig. 3A and B).
In this study, 71% (71/90) of Ph+ patients received dasatinib, and the OS of adherers and non-adherers was (84.9% [95% CI, 76.0 to 93.8] vs. 49.6% [95% CI, 32.1 to 67.1]), while 29% (19/90) received other TKIs with the OS of adherers and non-adherers was (73.3% [95% CI, 55.7 to 90.9] vs. 20.8% [95% CI, 2.4 to 39.2]). The adherence rate in the dasatinib and other TKIs group was 69.0% and 63.2%.
Allowing for patient age may be retained as a predictor for non-adherence, we divided the whole cohort into four groups: adherers in adolescence, adherers in non-adolescence (age ≤ 10 years), non-adherers in adolescence and non-adherers in non-adolescence, the OS were 85.3% (74.7%–95.9%), 81.0% (70.3%–91.7%), 54.4% (38.8%–70%), and 23.1% (4.2%–42%), respectively (p for trend=0.078) (Fig. 4).
3. Predictors for PFS and OS in Ph+ ALL patientsResults of the multivariate analysis indicated that a higher MMAS-8 score was with significantly lower risks in predicting PFS and OS in all Ph+ ALL patients ([HR, 0.657; 95% CI, 0.477 to 0.904; p=0.010 for PFS] and [HR, 0.650; 95% CI, 0.440 to 0.961; p=0.031 for OS]), which revealed that adherence of TKIs was closely correlated with the prognosis of children Ph+ ALL patients (Table 2). In addition, according to the results of univariable analysis, TKIs doses reduction during treatment showed a 3.8-fold higher risk for the OS of all patients (HR, 3.838; 95% CI, 1.216 to 12.118; p=0.022).
4. Adverse eventsRegarding safety, Table 3 showed the adverse events (AEs) of all included patients. The patients in the two groups were comparable with respect to AEs. In this study, all patients who experienced TKIs non-adherence (interruption or dose reduction) were owing to AEs, and most of them were pancytopenia. What’s more, fever, rash and musculoskeletal pain were the common AEs in patients taking TKIs.
DiscussionAs yet, this is the first study to investigate the patients’ oral TKIs adherence for children with Ph+ ALL during an up to two-and-a-half years-long therapy phase and reveal the influence of TKIs adherence on the prognosis of these patients. The results indicated that poor adherence to TKIs influenced the prognosis of Ph+ ALL children with significantly lower PFS and OS.
In patients with potentially life-threatening chronic illnesses (e.g., chronic myeloid leukemia [CML]), poor medication adherence share a common experience in clinical practice and is a major source of frustration for physicians. Based on previous reports, the non-adherence rate was demonstrated in 15%–30% of adult CML patients [15]. Compared with CML patients, Ph+ ALL children usually experienced multiple medications and extended periods of symptomatic remission, which contribute to being more vulnerable to poor TKIs adherence. Although previous studies have reported that intentional non-adherence to maintenance treatment rarely occurs (8%) in pediatric ALL, repeated forgetfulness is noted in 15% of these patients. In this study, 23.3% of Ph+ ALL children were regarded as non-adherers, which was higher than all childhood ALL patients from previous reports [16]. This inconsistency largely stems from the concomitant use of TKIs, methotrexate and mercaptopurine in this particular cohort. Although generally well tolerated, AEs were also observed in some patients receiving all generations of TKIs. Allowing for drug toxicity, transient treatment interruption and dose reduction were frequently required during TKIs treatment. It is possible that reduced dose intensity may result in poor efficacy of therapy.
Younger age is another well-known challenge to compliance in pediatric patients with malignancies because of increased risk-taking behaviours [17]. These patients were more likely to suffer from AEs and have difficulty taking the TKIs regimens, which may influence their prognosis. In this study, there existed little difference in OS between adolescents and non-adolescence adherers. Surprisingly, a worse prognosis was found in non-adolescence compared to adolescents in all non-adherers. Thus, younger non-adherers were more vulnerable to TKIs disruption.
Allowing for the sociodemographic and clinical characteristics were similar between patients in the two groups, the major barrier to TKI adherence is not the family income or parental education level. So we further analysed the eight items in the MMAS-8 questionnaire, and the results of the multivariate analysis revealed that the item “Have you ever cut back or stopped taking your medication without telling your doctor because you felt worse when you took it.” was the single factor which significantly correlated with TKI adherence. In view of this, the patients or caregivers should talk to their doctor before stopping taking any medicine, and another TKI treatment may work just as well and have fewer side effects.
Allowing for without a gold standard, any mathematical method used to compute medication adherence needs to be clearly defined. However, the assessment of adherence may differ greatly among studies and should be considered. Previous studies have demonstrated that the medication possession ratio (MPR) is highly influenced by the observation period and oversupply. Thus, some studies that used MPR present different formulas with poor specifications [18,19]. Consequently, each study was standalone, and further investigation was still warranted to assess the efficacy of the separate method. As regards criterion validity, the MMAS-8 scale was relatively simple and practical to use in clinical settings to assess compliance with drug regimens. It displayed constructive facts, with a clear trend toward a one-factor solution [20]. The MMAS-8 scale in this study showed significant correlations with patients’ positive attitudes toward their TKIs regimen, suggesting that the MMAS-8 scale could better reflect the treatment adherence in pediatric Ph+ ALL patients. Other than the MMAS-8 scale, we did not observe days of TKIs interruption or dose reduction significantly impacting the prognosis according to the multivariable Cox regression. However, allowing for the limited sample size of this cohort in such a subset analysis, these suggestions should be taken with extreme caution.
This study had several limitations. Firstly, although the MMAS-8 scale is a valid standard for measuring medication adherence, all the questionnaires applied are self-reports of caregivers, which carries a potential risk of misstatement or response biases. To minimize the bias in this study, all patients’ situation of drug ingestion was double-checked when they visited clinics or were re-admitted. Secondly, we only used one method to assess TKIs adherence. It should be noted that many ways are currently available for measuring compliance, on the account that each method is double-edged, and no method has been regarded as the gold standard.
In summary, TKIs adherence during treatment significantly impacts the survival of pediatric Ph+ ALL patients. And non-adherers with age ≤ 10 years were more vulnerable to TKIs disruption. Within this clinical reality, the practices associated with TKIs adherence should be emphasized in children with Ph+ ALL. The cumulative monthly TKIs dose should be especially emphasized to patients with age ≤ 10 years, which may result in an inferior achievement of relevant treatment milestones. Further studies with a more extensive study population and longer follow-up time were warranted to determine how the interruption of TKIs treatment or dose reduction due to toxicity affects the prognosis among this population.
NotesEthical Statement The study design and methods complied with the Declaration of Helsinki and were approved by the ethics committee of the Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College (IRB number: IIT2020020-EC-1). Informed consent was obtained from all subjects. AcknowledgmentsThe MMAS-8 Scale, content, name, and trademarks are protected by US copyright and trademark laws. Permission for use of the scale and its coding is required. A license agreement is available from MMAR, LLC., www.moriskyscale.com.
Fig. 1Correlation between total scores on the eight-item Morisky medication adherence scale (MMAS-8) and days of tyrosine kinase inhibitors (TKIs) interruption [11,13,14]. The MMAS-8 Scale, content, name, and trademarks are protected by US copyright and trademark laws. Permission for use of the scale and its coding is required. A license agreement is available from MMAR, LLC., www.moriskyscale.com. 
Fig. 2(A) Progression-free survival analysis in children with chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) in adherence versus non-adherence arms. (B) Overall survival analysis in children with Ph+ ALL in adherence vs. non-adherence arms. 
Fig. 3(A) Progression-free survival analysis in children with chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) who completed the treatment in adherence versus non-adherence arms. (B) Overall survival analysis in children with Ph+ ALL who completed the treatment in adherence vs. non-adherence arms. 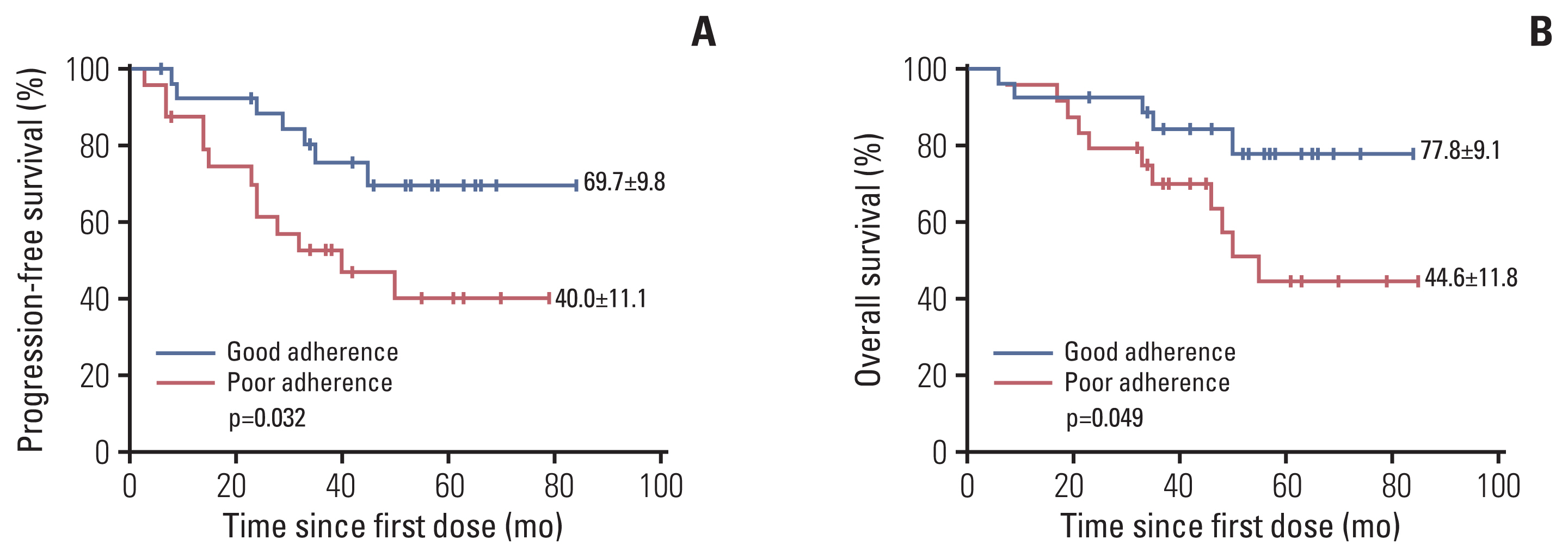
Fig. 4Overall survival analysis in children with chromosome-positive acute lymphoblastic leukemia from adherers vs. non-adherers in adolescence and non-adolescence. 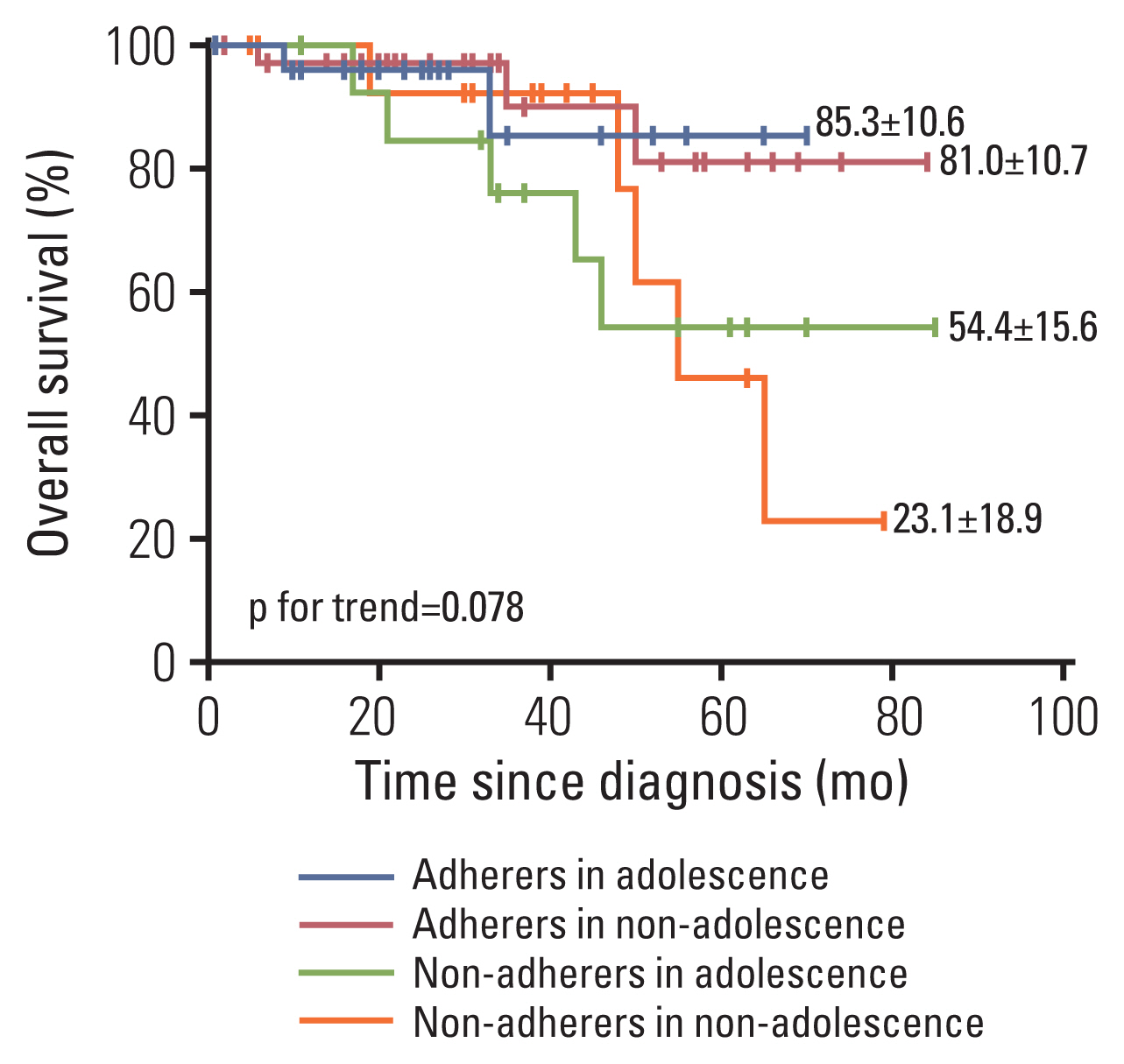
Table 1Clinical characteristics of all included childhood Ph+ ALL patients Permission for use of the scale and its coding is required. A license agreement is available from MMAR, LLC., www.moriskyscale.com. Values are presented as number (%) or median (range). ALL, acute lymphoblastic leukemia; CNY, Chinese Yuan RMB; MRD, minimal residual disease; Ph+, chromosome-positive; TKI, tyrosine kinase inhibitor. Table 2Multivariable model for predicting risk factors of survival in Ph+ ALL patients Table 3Adverse events in childhood Ph+ ALL patients during TKIs treatment References2. Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37:3377–91.
3. Leoni V, Biondi A. Tyrosine kinase inhibitors in BCR-ABL positive acute lymphoblastic leukemia. Haematologica. 2015;100:295–9.
4. Shen S, Chen X, Cai J, Yu J, Gao J, Hu S, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 2020;6:358–66.
5. Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi X, Pulsipher MA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622. J Clin Oncol. 2018;36:2306–14.
6. Jabbour E, Haddad FG, Short NJ, Kantarjian H. Treatment of adults with Philadelphia chromosome-positive acute lymphoblastic leukemiafom intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 2022;8:1340–8.
7. De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169:30–5.
8. Yang W, Cai J, Shen S, Gao J, Yu J, Hu S, et al. Pulse therapy with vincristine and dexamethasone for childhood acute lymphoblastic leukaemia (CCCG-ALL-2015): an open-label, multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021;22:1322–32.
9. Tang J, Yu J, Cai J, Zhang L, Hu S, Gao J, et al. Prognostic factors for CNS control in children with acute lymphoblastic leukemia treated without cranial irradiation. Blood. 2021;138:331–43.
10. Woodard L, Amspoker AB, Hundt NE, Gordon HS, Hertz B, Odom E, et al. Comparison of collaborative goal setting with enhanced education for managing diabetes-associated distress and hemoglobin A1c levels: a randomized clinical trial. JAMA Netw Open. 2022;5:e229975.
11. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–54.
12. Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64:255–7.
13. Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, et al. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 2017;377:733–44.
14. Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377:745–55.
15. Geissler J, Sharf G, Bombaci F, Daban M, De Jong J, Gavin T, et al. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143:1167–76.
16. Mancini J, Simeoni MC, Parola N, Clement A, Vey N, Sirvent N, et al. Adherence to leukemia maintenance therapy: a comparative study among children, adolescents, and adults. Pediatr Hematol Oncol. 2012;29:428–39.
17. Millot F, Claviez A, Leverger G, Corbaciglu S, Groll AH, Suttorp M. Imatinib cessation in children and adolescents with chronic myeloid leukemia in chronic phase. Pediatr Blood Cancer. 2014;61:355–7.
18. Govani SM, Noureldin M, Higgins PD, Heisler M, Saini SD, Stidham RW, et al. Defining an optimal adherence threshold for patients taking subcutaneous anti-TNFs for inflammatory bowel diseases. Am J Gastroenterol. 2018;113:276–82.
|
|
|||||||||||||||||||||||||||||||||||||||