AbstractPurposeWe investigated the consistent efficacy and safety of eflapegrastim, a novel long-acting granulocyte-colony stimulating factor (G-CSF), in Koreans and Asians compared with the pooled population of two global phase 3 trials.
Materials and MethodsTwo phase 3 trials (ADVANCE and RECOVER) evaluated the efficacy and safety of fixed-dose eflapegrastim (13.2 mg/0.6 mL [3.6 mg G-CSF equivalent]) compared to pegfilgrastim (6 mg based on G-CSF) in breast cancer patients who received neoadjuvant or adjuvant docetaxel/cyclophosphamide. The primary objective was to demonstrate non-inferiority of eflapegrastim compared to pegfilgrastim in mean duration of severe neutropenia (DSN) in cycle 1, in Korean and Asian subpopulations.
ResultsAmong a total of 643 patients randomized to eflapegrastim (n=314) or pegfilgrastim (n=329), 54 Asians (29 to eflapegrastim and 25 to pegfilgrastim) including 28 Koreans (14 to both eflapegrastim and pegfilgrastim) were enrolled. The primary endpoint, DSN in cycle 1 in the eflapegrastim arm was non-inferior to the pegfilgrastim arm in Koreans and Asians. The DSN difference between the eflapegrastim and pegfilgrastim arms was consistent across populations: −0.120 days (95% confidence interval [CI], −0.227 to −0.016), −0.288 (95% CI, −0.714 to 0.143), and −0.267 (95% CI, −0.697 to 0.110) for pooled population, Koreans and Asians, respectively. There were few treatment-related adverse events that caused discontinuation of eflapegrastim (1.9%) or pegfilgrastim (1.5%) in total and no notable trends or differences across patient populations.
IntroductionCancer remains a leading cause of death worldwide, although cancer therapeutics including cytotoxic chemotherapy, targeted therapy, and immunotherapy have recently been in great progress [1]. Neutropenia has presented a major challenge in cancer treatment since the introduction of cytoxotic chemotherapy in the 1950s. The advent of recombinant human granulocyte-colony stimulating factor (G-CSF) reduced the incidence and duration of severe neutropenia (DSN) with myelosuppressive chemotherapy, along with the complications [2–4]. The development of a long-acting G-CSF products offers a more convenient dosing schedule to patients, improving compliance, and possibly greater clinical efficacy.
Eflapegrastim (Rolontis) is a long-acting G-CSF that has been developed to reduce the severity and duration of severe neutropenia, as well as complications of neutropenia, associated with the use of myelosuppressive anti-cancer drugs. Eflapegrastim is a novel biologic and not a biosimilar to any currently marketed G-CSF product. Instead, eflapegrastim is a unique recombinant protein expressed by Escherichia coli, which consists of a human G-CSF analog (HM10411, rh G-CSF) and a human IgG4 Fc fragment (HMC001) that are covalently coupled by a single 3.4 kDa polyethylene glycol linker. The recommended dose of eflapegrastim is a fixed dose of 13.2 mg/0.6 mL (3.6 mg G-CSF equivalent) administered by subcutaneous injection once-per-chemotherapy-cycle approximately 24 hours after chemotherapy administration. This dosing regimen was shown to be well tolerated and associated with a low rate and short DSN comparable to pegfilgrastim in patients treated with myelosuppressive chemotherapy in two phase III trials (ADVANCE, NCT02643420 and RECOVER, NCT02953340) [5,6]. These two multi-national, randomized, open-label active-controlled phase III trials were conducted as pivotal studies for the Biologics License Application submission in the United States and Korea. The trials were performed to demonstrate the non-inferiority of eflapegrastim compared with pegfilgrastim in early breast cancer (EBC) patients receiving adjuvant or neoadjuvant docetaxel and cyclophosphamide (TC) across six countries: Canada, Hungary, India, Korea, Poland, and United States.
However, there have been no independent studies or analyses regarding efficacy and safety of eflapegrastim in Asian patients including Koreans. Therefore, we performed subgroup analyses of two phase III trials with eflapegrastim, ADVANCE and RECOVER trials in a subpopulation, focusing on Asian ethnicity including Koreans.
Materials and Methods1. Study design and study populationADVANCE and RECOVER trials were international, multicenter, open-label, randomized, phase 3 studies comparing eflapegrastim versus pegfilgrastim in patients with EBC who planned to receive adjuvant or neoadjuvant TC. Female or male patients were eligible if they were at least 18 years of age with newly diagnosed histologically confirmed EBC who were candidates to receive adjuvant or neoadjuvant TC chemotherapy. They had adequate hematological, renal, and hepatic function and Eastern Cooperative Oncology Group performance status ≤ 2. Exclusion criteria included concurrent adjuvant cancer therapy (chemotherapy other than TC, radiation therapy, immunotherapy, biologic therapy other than the trial specified therapies), locally recurrent/metastatic breast cancer and history of participation in any investigational drug trial with filgrastim, pegfilgrastim, or other G-CSF products within 12 months prior to the administration of study drug (eflapegrastim or pegfilgrastim). The Korean population used for the subgroup analyses was defined as the patients enrolled at study sites in Korea; Asian population included Korean population and the patients of Asian ethnicity enrolled at global sites.
2. ProceduresThe two phase 3 studies (ADVANCE, RECOVER) were identical with respect to study design, patient population, dosage regimen, primary endpoint, statistical hypothesis, and methodology except for the statistical power and total number of patients. The phase 2 dose-ranging study (SPI GCF 12 201), which included breast cancer patients with characteristics similar to patients in the two phase 3 studies who were treated with either eflapegrastim (n=112) or pegfilgrastim (n=36), provided supportive efficacy data and to determine the fixed dose of eflapegrastim used in the phase 3 studies [7]. ADVANCE study was primarily conducted in the United States (77 sites) and Canada (3 sites) with two sites in Korea. RECOVER study was also conducted primarily within the United States (49 sites) and Canada (2 sites) but expanded to other regions; Hungary (6 sites), Poland (7 sites), India (2 sites), and Korea (8 sites). Eligible patients were randomized at a 1:1 ratio stratified by study site using a permuted block design. All randomized participants received either pegfilgrastim 6 mg/0.6 mL or eflapegrastim 13.2 mg/0.6 mL on day 2 of each chemotherapy cycle, 24 to 26 hours after TC administration up to 4 cycles (21-day cycle) plus a 12-month follow-up from the last dose. The phase 3 study design diagram is presented in Fig. 1. Absolute neutrophil count (ANC) was monitored on day 1 and days 4 to 15 in cycle 1. In ADVANCE and RECOVER studies, the protocol prohibited the use of additional growth factors (filgrastim or pegfilgrastim) during the treatment period and prophylactic antibiotics were not routinely used. In cycles 2 to 4, all patients must have blood samples drawn on day 1 (prior to chemotherapy administration), on days 4, 7, 10, and 15 (±1 day for each collection), and at the end-of-treatment visit. If the participating site was notified that the ANC is ≤ 1.0×109/L at any time in cycles 2 to 4, then daily complete blood cell count was required until the ANC is ≥ 1.5×109/L, after reaching nadir, but blood samples must still be drawn on days 4, 7, 10, and 15. As applicable, patients who had received at least one dose of study drug was followed for 12 months after the last dose of study treatment for safety follow-up. Since the efficacy endpoints consisted of objective hematologic laboratory measurements, there was a low potential for bias in the assessment of efficacy despite the open-label study design. Nevertheless, a data review and dissemination plan was implemented for each study to limit the knowledge of the efficacy results to a small subset of study team who does not have any interaction with the investigators and sites.
3. Statistical analysisThe primary objective of both phase 3 studies (ADVANCE, RECOVER) was to compare the efficacy of eflapegrastim with pegfilgrastim in patients with EBC receiving TC, as measured by DSN in cycle 1. The primary efficacy endpoint, DSN in cycle 1, was defined as the number of days of severe neutropenia (ANC < 0.5×109/L) from the first occurrence of an ANC below the threshold. Key secondary objectives were to compare the following parameters resulting from treatment with eflapegrastim with those resulting from treatment with pegfilgrastim: the time to ANC recovery in cycle 1, depth of ANC nadir in cycle 1, incidence of febrile neutropenia in cycle 1, and safety. Safety was assessed by the incidence of adverse events (AEs) graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE ver. 4.03).
The efficacy analyses were primarily based on the intent-to-treat (ITT) population including all randomized patients in compliance with ITT principle. The per protocol population as one of sensitivity analyses were defined as all patients in the ITT population with no important protocol deviation in cycle 1. The safety analysis population included all randomized patients who received at least one dose of any protocol-specified drug (TC or eflapegrastim or pegfilgrastim). Along with the global pooled patient population, subpopulation analyses for Korean and Asian patients were performed to investigate the consistency of global results in the subpopulation. All data analyses were carried out using the SAS system ver. 9.3 software package (SAS Institute Inc., Cary, NC).
Sample size estimates were based on a non-inferiority design and the margin of non-inferiority was 0.62 days for both phase 3 studies assuming the true difference between the means was assumed to be 0.0 days. In the ADVANCE study, the pooled standard deviation (SD) of the DSN was assumed to be 2.0 days and sample sizes of 200 per treatment were determined to provide 87% power to detect non-inferiority using a one-sided, two-sample t-test at 2.5% level of significance. Similarly, in the RECOVER study, 109 per treatment arm was determined to provide 90%, 86%, and 81% power to detect non-inferiority assuming the pooled SD of the DSN was 1.4, 1.5, or 1.6 days, respectively. In particular, the number of Korean patients in RECOVER study was determined at least 10% of total target patients considering to provide about 80% of probability to assure consistency for the Korea region, conditioning on the significant effect of overall global trial as Korean bridging study.
Results1. Patient characteristics
Fig. 2 presents patient disposition of each population by treatment arm based on the pooled data. Treatment groups were well balanced with respect to relevant baseline demographic and disease characteristics in each of the subgroup patients and the total as shown in Table 1. In the pooled data, most patients in each treatment group were female (> 99%). The median weights in each treatment group for Korean (57.4 kg and 58.5 kg, respectively) and Asian patients (56.3 kg and 58.9 kg, respectively) were lower than the non-Asian patients (79.0 kg and 78.0 kg, respectively). The disease stage at diagnosis was evenly distributed overall and most of the patients were stage I or II.
2. EfficaciesAmong a total of 643 patients randomized to eflapegrastim (n=314) or pegfilgrastim (n=329), 54 Asians (29 to eflapegrastim and 25 to pegfilgrastim) including 28 Koreans (14 for both eflapegrastim and pegfilgrastim) were included for primary analysis. The primary endpoint, the mean DSN in cycle 1 (SD) was 0.24 days (0.581) for eflapegrastim arm and 0.36 days (0.789) for pegfilgrastim in the pooled ITT population (Table 2). The difference between groups was −0.12 days (95% confidence interval [CI], −0.227 to −0.016) which demonstrated non-inferiority of eflapegrastim over pegfilgrastim in the pooled patients. In the subgroup analysis of Korean patients, the mean DSN difference between groups was −0.288 days (95% CI, −0.714 to 0.143). Similarly, the mean DSN differences between groups in Asian and non-Asian patients were −0.267 days (95% CI, −0.697 to 0.110) and −0.107 days (95% CI, −0.222 to 0.002). The 95% CIs are relatively wider for Koreans (n=28) and Asians (n=54) due to small number of patients compared with the total patients (n=643) but the upper limits of the 95% confidence intervals were all less than 0.62 days, which supported consistent non-inferiority across patient groups irrespective of the number of patients included. In addition, as presented in Fig. 3 and S1 and S2 Figs., the forest plots by analysis method indicate robust non-inferiority of eflapegrastim over pegfilgrastim in DSN in cycle 1.
In the pooled analysis, secondary efficacy endpoints were also evaluated between treatment arms by patient groups (Table 3). Firstly, difference in time to ANC recovery in cycle 1 was −0.714 (95% CI, −5.627 to 4.198), 0.023 (95% CI, −3.536 to 3.583), and −0.103 (95% CI, −1.059 to 0.852) for Korean, Asian and all patients, respectively. Secondly, ratio of depth of ANC nadir (×109/L) in cycle 1 of eflapegrastim over pegfilgrastim was all greater than 1, in favor of eflapegrastim though those were not statistically significant. Lastly, the overall incidence of febrile neutropenia in cycle 1 was low in both phase 3 studies, five (1.6%) for eflapegrastim treated arm and six (1.8%) in pegfilgrastim treated arm in the pooled data and the difference between treatment arms was not significant (nominal p=1.000). There was no febrile neutropenia in cycle 1 for eflapegrastim treated Asian patients and there were 2 (8.0%) cases for pegfilgrastim treated Asians, of which one (7.1%) was Korean patient. Overall, the differences in the key secondary endpoints between treatment groups were not statistically significant but the differences were in favor of eflapegrastim treated arm in Koreans, Asians and all patients (Table 3).
3. Adverse eventsThe overall summary of AEs during the treatment period is presented by treatment arms and patient groups in Table 4. Most of the patients (≥ 96%) experienced treatment emergent adverse events (TEAEs) but most AEs were attributed to cytotoxic chemotherapy and the safety profile of eflapegrastim was similar to that of pegfilgrastim in all patient groups (Table 4). TEAEs of grade 3 or higher severity were experienced by a similar percentage of patients receiving eflapegrastim and pegfilgrastim (73.0% vs. 72.8%) in non-Asian patients while 92.9% vs. 78.6% in Korean patients and 86.2% vs. 64% in Asian patients. The incidence of drug-related AEs was: for all grades, 77.2% with eflapegrastim versus 68.1% with pegfilgrastim and for grade 3/4, 16.8% with eflapegrastim versus 10.0% with pegfilgrastimin non-Asian patients (Table 4), which is numerically higher in the eflapegrastim arm and such trend is indicated in Korean and Asian patient groups. In more detail, in Table 5 we listed up each AEs related to eflagrastim or pegfilgrastim occurring in ≥ 10% of patients in any subgroup. When arthralgia, back pain, bone pain, and myalgia were all grouped into musculoskeletal pain, which is a common AE associated with treatment with myeloid growth factors, incidence differences of grade 3/4 drug-related AEs between eflapegrastim and pegfilgrastim were attributed to musculoskeletal pain. Grade 3 musculoskeletal pain was 10.3% with eflapegrastim vs. 0% with pegfilgrastim in Asian population and 7.4% with eflapegrastim vs. 1.7% with pegfilgrastim in non-Asian population. However, other AEs (grade 3/4) than musculoskeletal pain were similar between eflapegrastim and pegfilgrastim (Table 5). There were a few patients experienced in study drug–related serious AEs in both treatment arms and 2.1% vs. 1.3% for each non-Asian eflapegrastim and pegfilgrastim treatment arm discontinued treatment due to the study drug–related AEs. No patients died after receiving eflapegrastim. Two patients died within 35 (±5) days of the last dose of pegfilgrastim (1 due to cardiac arrest and 1 due to chronic obstructive pulmonary disease); two mortalities were not related to treatment with pegfilgrastim and they had no sign of leukocytosis. In general, the percentage of AEs for each treatment group in Korean and Asian patients seemed numerically higher than that of non-Asian patients but that was caused by the small number of patients in denominator. The distribution of incidence of AEs seems comparable between treatment groups across patient populations without clinically significant events.
The most frequent AEs (≥ 30% in either arm) were nausea, fatigue, alopecia, diarrhea, lymphocyte count decreased, bone pain, and neutropenia, most of which were due to the chemotherapy, especially the docetaxel chemotherapy. The increase in ANC over time was higher in the eflapegrastim group than in the pegfilgrastim group but mostly it was transient and returned to normal range at the end of the study visit (Fig. 4). Thirty-four cases of any grade white blood cell (WBC) count increased (i.e., leukocytosis), and three cases of grade 3 leukocytosis (WBC > 100×109/L) were reported in the non-Asian eflapegrastim group and 18 cases of any grade leukocytosis and three cases of grade 3 leukocytosis in the non-Asian pegfilgrastim group resulting one patient in each group withdraw the investigational product (IP) and one patient in the eflapegrastim group skipped IP dose of cycle 4. All patients recovered except four patients (3 in the eflapegrastim and 1 in the pegfilgrastim group) and two patients’ outcomes were unknown. There was no significant difference in the incidence of AEs between the groups.
DiscussionThe advent of pegfilgrastim, the first long-acting rhG-CSF, effectively diminished the risk of chemotherapy-induced neutropenia, with a more simplified way which is a once-per-chemotherapy-cycle option [8]. Results from the previous two pivotal phase III trials (ADVANCE, RECOVER) confirmed that eflapegrastim, new long-acting rhG-CSF adding Fc fragment to extend drug half-life and increase the distribution into the bone marrow, was non-inferior in efficacy and comparable in safety to pegfilgrastim [5,6]. However, outcomes by ethnicity have not previously been reported in these studies.
Consistency of treatment effect across regions is often a key issue in a multiregional clinical trial due to various differences in intrinsic and extrinsic factors [9]. Thus, prudent consideration to evaluate consistency in treatment effect across regions needs to be made from the design stage. In fact, some published trials indicated difference in treatment effects by region [10,11]. ADVANCE study was conducted in the United States, Canada, and the Republic of Korea but the majority of patients (97%) were enrolled in the United States and six (1.5%) Koreans and 18 (4%) Asians were enrolled. RECOVER study was considered to investigate whether treatment effect would be consistent between the entire population and the Korean subpopulation as Korean bridging study. With the expected proportion of Korean patients in the study, the probability of observing a consistent trend across Korean and Non-Korean region were calculated using the criteria of consistency based on the Japanese Ministry of Health, Labour, and Welfare guidance [12,13], which describes consistency in the sense that estimate of treatment effect needs to meet the prespecified criteria in the region of interest in addition to overall treatment in all regions combined. According to the guidance, the number of patients in Korean region was planned to have over 80% probability ensuring consistency, conditioning on the significant effect of overall global trial. Thus, if non-inferiority of eflapegrastim in the DSN for cycle 1 compared to pegfilgrastim was demonstrated in the overall global trial, subgroup analysis for Korean patients was to be performed. Obviously, the non-inferiority was demonstrated in the entire population of the RECOVER study and also in the subgroup analysis for Korean patients [5]. In this paper, the same analyses as those in each phase 3 study were performed based on the pooled data. The respective treatment groups in Korean, Asian and all patients were balanced with respect to relevant demographic characteristics. Eflapegrastim consistently presented non-inferiority to pegfilgrastim with respect to the DSN in cycle 1 in the primary efficacy analysis as well as sensitivity analyses in Korean, Asian patients and in all pooled patients from the two phase 3 studies (S1 and S2 Figs.). Thus, the robustness of eflapegrastim treatment effect across all subpopulations was supported along with the previously published results [5,6]. In addition, the mean and median values for the time to ANC recovery and the depth of ANC nadir in cycle 1 were also similar between the treatment arms for Korean, Asian, and all patients. The number of patients who experienced febrile neutropenia was very low in both treatment arms in cycle 1 from the two phase 3 studies; there was no statistically significant difference between the treatment arms in the secondary endpoints such as time to ANC recovery, depth of ANC nadir, and incidence of febrile neutropenia.
There were no notable trends or differences between treatment groups in the incidence of all grades or grade 3/4 TEAEs. Although drug-related AEs, in particular of grade 3/4 had a trend toward higher in the eflapegrastim arm in Asian and non-Asian population, this was caused by musculoskeletal pain. Higher incidence of grade 3 musculoskeletal pain with eflapegrastim may result from potentially stronger stimulation of bone marrow with eflapegrastim as shown in a preclinical study [14]. Fixed dose of pegfilgrastim 6 mg/0.6 mL or eflapegrastim 13.2 mg/0.6 mL were used regardless of body weight in both studies. The median body weight for Korean was 57.4 kg and for Asian patients 56.3 kg in eflapegrastim group which was more than 20 kg lower than the median body weight for non-Asian in eflapegrastim group (79 kg). This difference in body weight might cause more frequent musculoskeletal symptoms for Asian patients. Moreover, these musculoskeletal pains did not cause serious morbidities because these could be managed with pain medicine including weak opioids and non-steroidal anti-inflammatory drugs.
Although this study was designed to demonstrate non-inferiority as mentioned above, eflapegrastim had numerically better efficacy in the primary efficacy analysis, irrespectively of in Asians or non-Asians. Even though it is not considered statistically significant, we may suggest biologically plausible reasons for this. Eflapegrastim and pegfilgrastim had similar in vitro binding affinity but the FcRn fragment in eflapegrastim increased the uptake of the drug into bone marrow. In addition, eflapegrastim showed greater bone marrow exposure and retention, resulting in increased potency in animal model [14]. These findings also support eflapegrastim same-day administration with chemotherapy, and a clinical trial is underway (NCT04187898).
Taken together, although not prespecified or powered to confirm treatment effects in each subpopulation, the pooled analyses based on the two global phase 3 studies may suggest consistently non-inferior efficacy and comparable safety results between eflapegrastim and pegfilgrastim in early-stage breast cancer patients receiving TC chemotherapy across Korean, Asian and all patients. Thus, these consistent findings support that eflapegrastim would be a new reasonable option for the target Asian patients including Koreans.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website https://www.e-crt.org.
NotesEthical Statement The study protocols were approved by the Institutional Review Board and/or Ethics Committee of each participating center and local regulatory. All patients gave written informed consent before any study-related procedure was performed. Author Contributions Conceived and designed the analysis: Moon YW, Im SA. Collected the data: Moon WY, Kim SK, Lee KS, Lee MH, Park YH, Park KH, Kim GM, Lim S, Lee SA, Im SA. Contributed data or analysis tools: Moon WY, Kim SK, Baek E, Choi JD, Han H, Baek S. Performed the analysis: Moon WY, Kim SK, Baek E, Choi JD, Han H, Baek S, Im SA. Wrote the paper: Moon YW, Baek E, Han H, Choi JD, Baek S, Im SA. Review the manuscript: Moon YW, Kim SK, Lee KS, Lee MH, Park YH, Park KH, Kim GM, Lim S, Lee SA, Choi JD, Baek E, Han H, Baek S, Im SA. Fig. 1Study design diagram. G-CSF, granulocyte-colony stimulating factor; IV, intravenously; SC, subcutaneously. 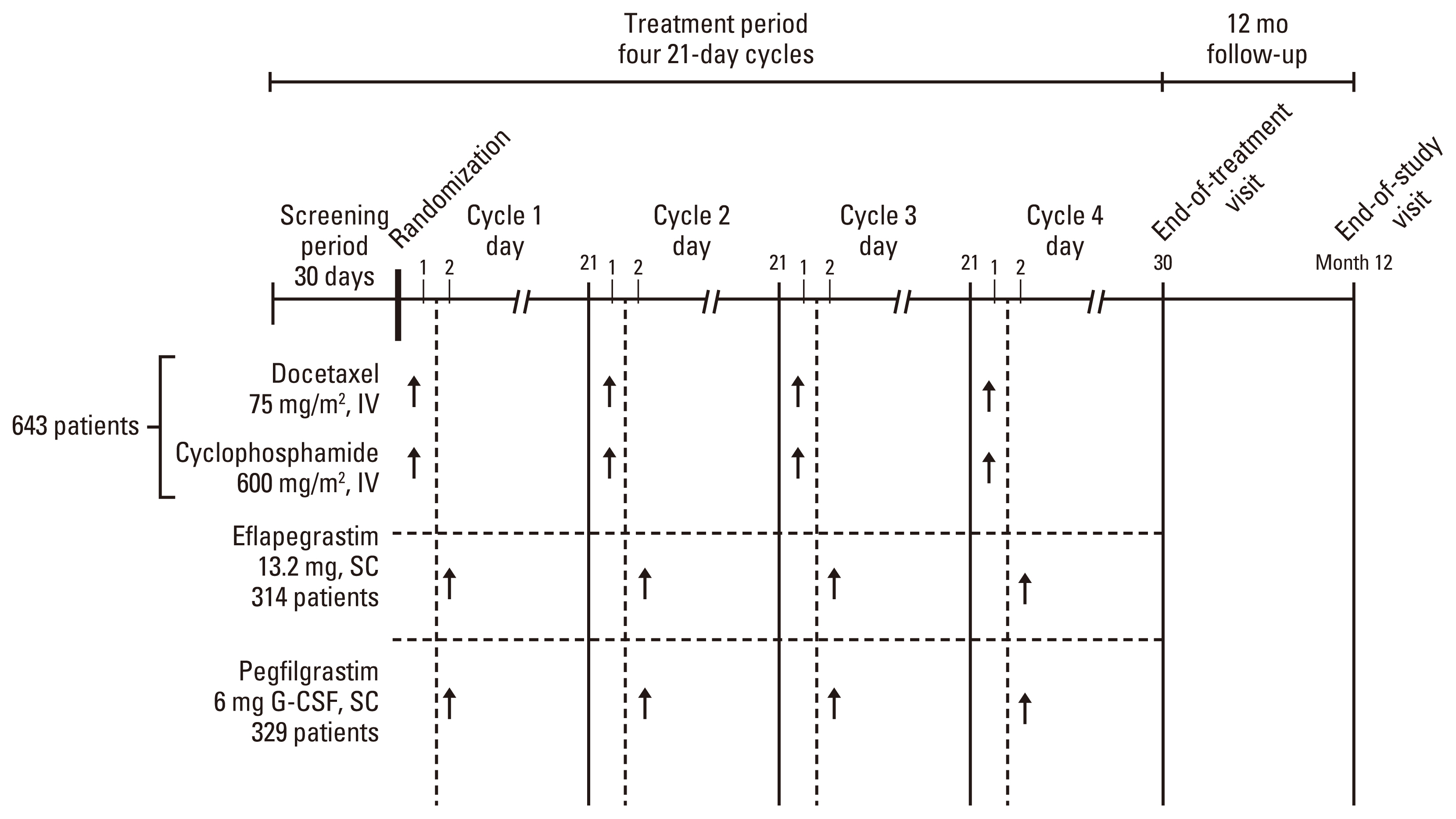
Fig. 2Patient disposition diagram (ADVANCE, RECOVER, and pooled data). The number of Asian patients includes Korean patients and non-Korean Asian patients. Total three patients (non-Asian; Eflapegrastim 1, Pegfilgrastim 2) was randomized, however, never received any study drug. One patient (non-Asian) was randomized to Pegfilgrastim but was given eflapegrastim on cycle 1 day 2. This patient was assigned to Eflapegrastim Safety population. ITT, intent-to-treat. 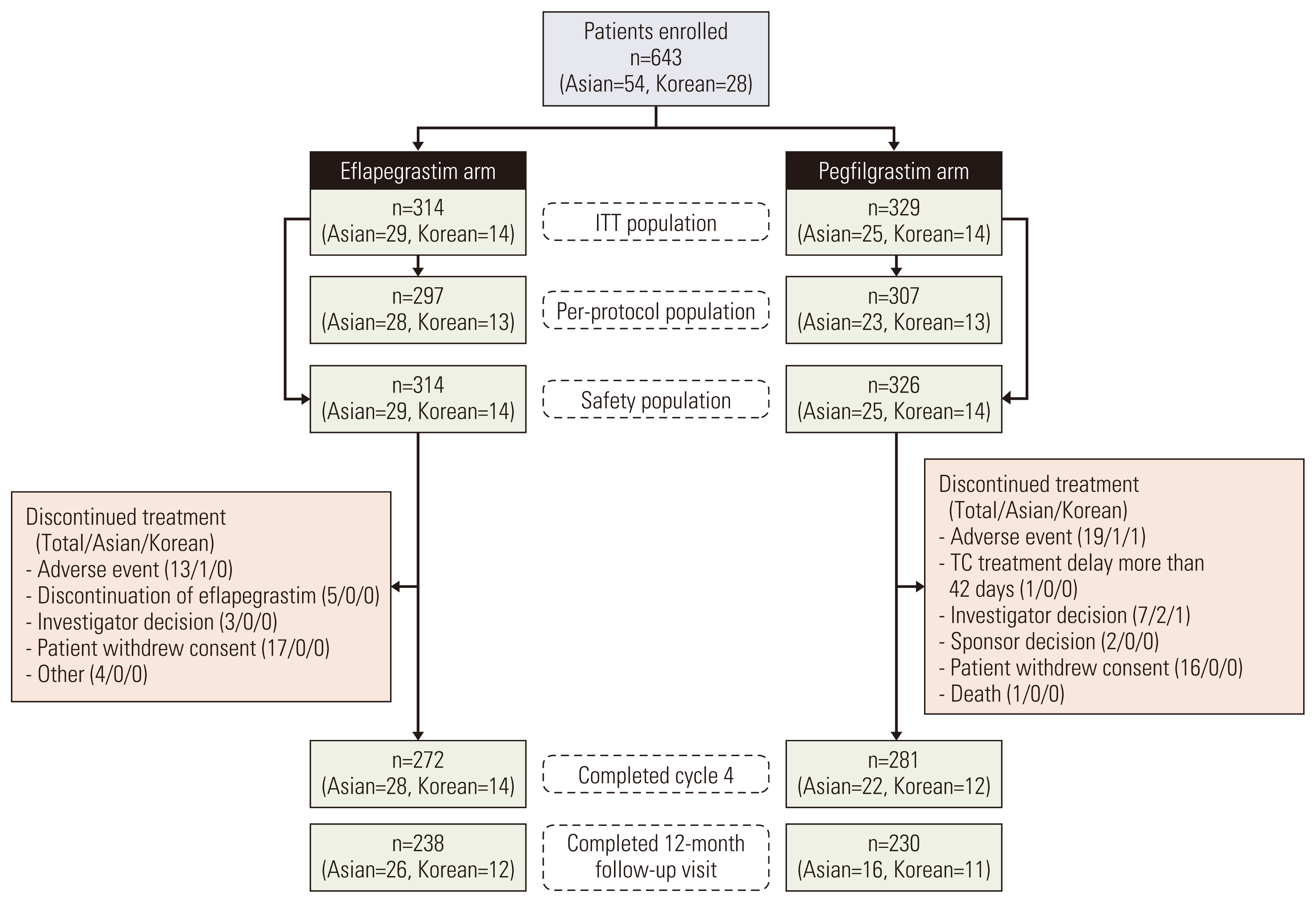
Fig. 3Subgroup analyses of primary efficacy. a, primary analysis in intention-to-treat (ITT) population; b, sensitivity analysis in per protocol (PP) population. CI, confidence interval. 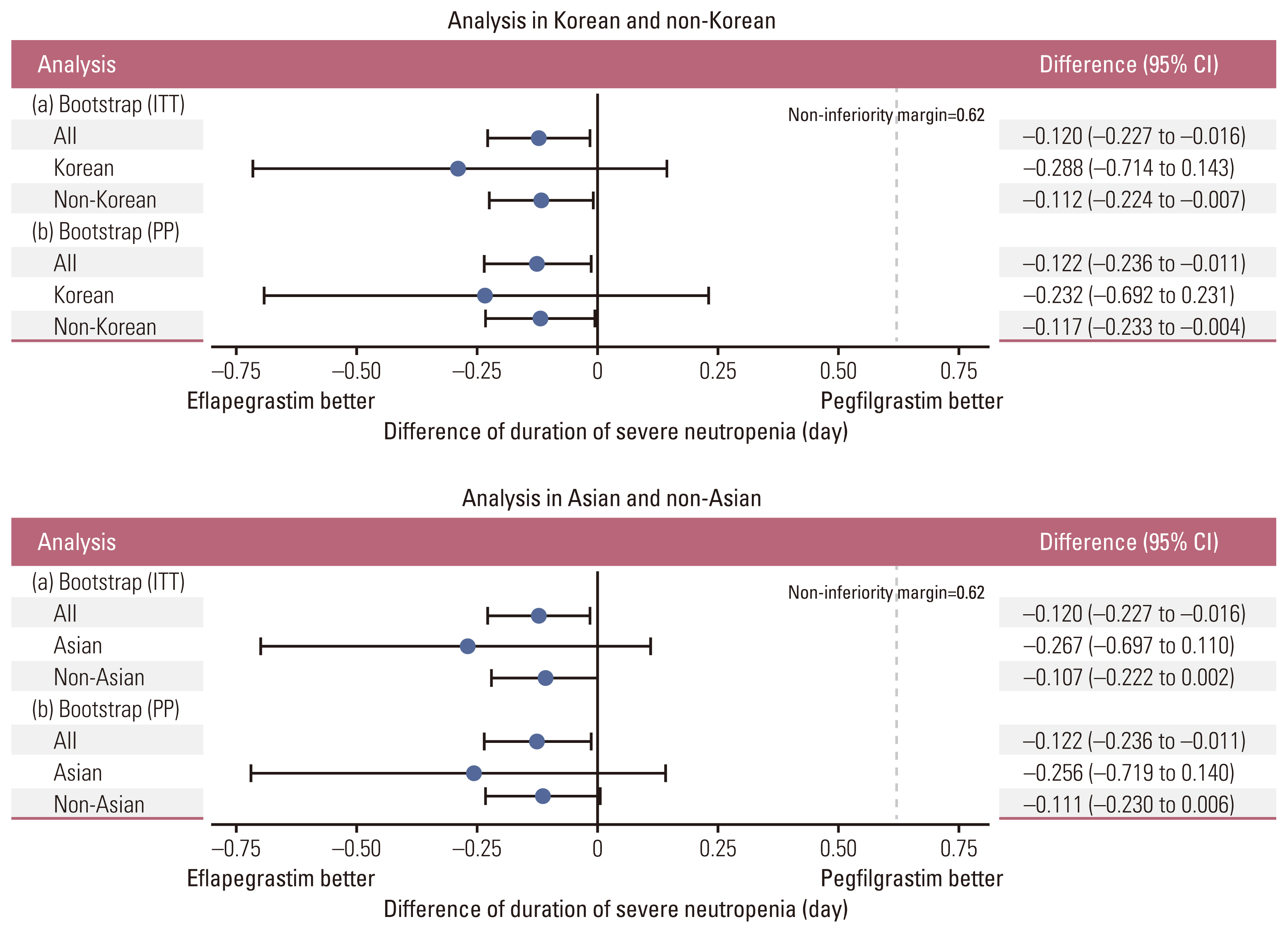
Fig. 4Plot of mean (±standard error) absolute neutrophil count (ANC) over time by treatment in cycle 1. ANC values measured at day 1, day 4 through day 15, and day 22 were included. Day 1 ANC values of next cycle were depicted with values on day 22. SPI-2012 indicates eflapegrastim. 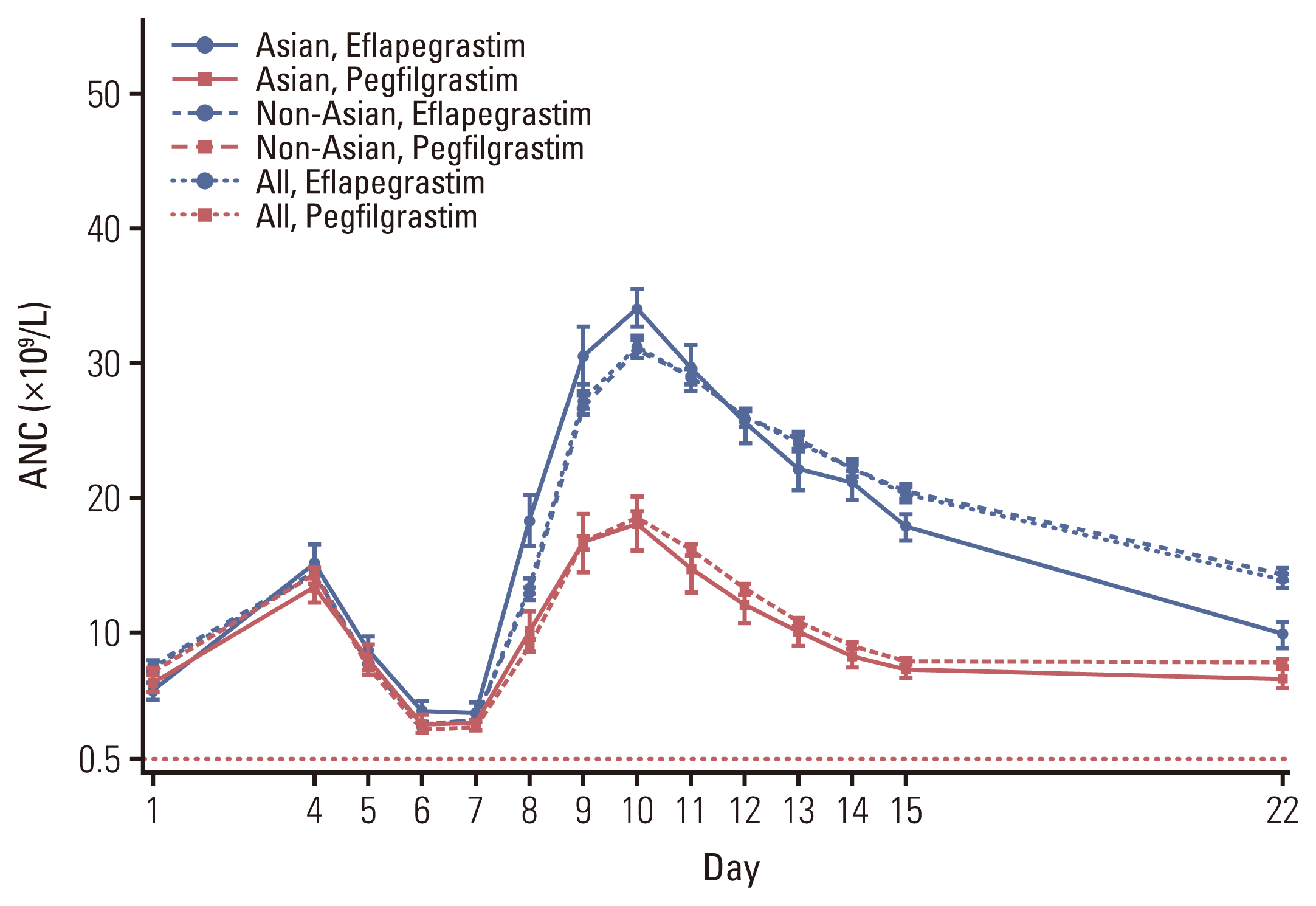
Table 1Patient characteristics Table 2Primary efficacy analysis
Table 3Secondary efficacy endpoints
ANC, absolute neutrophil count; CI, confidence interval; ITT, intent-to-treat; SD, standard deviation. a) Time to ANC recovery in cycle 1, defined as the time from chemotherapy administration until the patient’s ANC increases to ≥ 1.5×109/L after the expected nadir. For patients with ANC value ≥ 1.5×109/L at all times, Time to ANC recovery will be assigned to a value of 0, b) Time to ANC recovery was 0 day in 6 patients, 7 days in 6 patients and 8 days in 2 patients for pegfilgrastim arm, whereas, it was 0 day in more than half (8 out of 14) of eflapegrastim treated Korean patients during the cycle 1. Thus it was calculated that the median time to ANC recovery was 7 days for pegfilgrastim arm versus 0 day for eflapegrastim in Korean patients during the cycle 1, Table 4Overall summary of adverse events Table 5Adverse events related to eflapegrastim or pegfilgrastim occurring in ≥ 10% of patients in any subgroup
References1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27.
2. National Comprehensive Cancer Network. Breast cancer, version 2 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2017. [cited 2023 Jan 16]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32.
4. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–67.
5. Cobb PW, Moon YW, Mezei K, Lang I, Bhat G, Chawla S, et al. A comparison of eflapegrastim to pegfilgrastim in the management of chemotherapy-induced neutropenia in patients with early-stage breast cancer undergoing cytotoxic chemotherapy (RECOVER): p phase 3 study. Cancer Med. 2020;9:6234–43.
6. Schwartzberg LS, Bhat G, Peguero J, Agajanian R, Bharadwaj JS, Restrepo A, et al. Eflapegrastim, a long-acting granulocyte-colony stimulating factor for the management of chemotherapy-induced neutropenia: results of a phase III trial. Oncologist. 2020;25:e1233–41.
7. Barrett JA, Greene D, Lakshmikanthan S, Kolli P, Chawla S, Lebel F. Justification for a fixed dose of eflapegrastim, a long-acting G-CSF, in patients receiving docetaxel-cyclophosphamide chemotherapy. J Clin Pharmacol. 2021;61:204–10.
8. Arvedson T, O’Kelly J, Yang BB. Design rationale and development approach for pegfilgrastim as a long-acting granulocyte colony-stimulating factor. BioDrugs. 2015;29:185–98.
9. Chen J, Quan H, Gallo P, Menjoge S, Luo X, Tanaka Y, et al. Consistency of treatment effect across regions in multiregional clinical trials, part 1: design considerations. Drug Inf J. 2011;45:595–602.
10. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57.
11. Akkerhuis KM, Deckers JW, Boersma E, Harrington RA, Stepinska J, Mahaffey KW, et al. Geographic variability in outcomes within an international trial of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes: results from PURSUIT. Eur Heart J. 2000;21:371–81.
12. Kawai N, Chuang-Stein C, Komiyama O, Li Y. An approach to rationalize partitioning sample size into individual regions in a multiregional trial. Drug Inf J. 2008;42:139–47.
13. Ministry of Health, Labour and Welfare. Basic Principles on Global Clinical Trials [Internet]. Tokyo: Pharmaceuticals and Medical Devices Agency; 2007. [cited 2023 Jan 16]. Available from: http://www.pmda.go.jp/files/000157900.pdf
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||