AbstractPurposeIn sentinel lymph node (SLN) biopsy (SLNB) during breast cancer surgery, SLN mapping using dye and isotope (DUAL) may have lower false-negative rates than the dye-only (DYE) method. However, the long-term outcomes of either method are unclear. We aimed to compare long-term oncological outcomes of DYE and DUAL for SLNB in early breast cancer.
Materials and MethodsThis retrospective single-institution cohort study included 5,795 patients (DYE, 2,323; DUAL, 3,472) with clinically node-negative breast cancer who underwent SLNB and no neoadjuvant therapy. Indigo carmine was used for the dye method and Tc99m-antimony trisulfate for the isotope. To compare long-term outcomes, pathologic N0 patients were selected from both groups, and propensity score matching (PSM), considering age, pT category, breast surgery, and adjuvant treatment, was performed (1,441 patients in each group).
ResultsThe median follow-up duration was 8.7 years. The median number of harvested sentinel nodes was 3.21 and 3.12 in the DYE and DUAL groups, respectively (p=0.112). The lymph node–positive rate was not significantly different between the two groups in subgroups of similar tumor sizes (p > 0.05). Multivariate logistic regression revealed that the mapping method was not significantly associated with the lymph node–positive rate (p=0.758). After PSM, the 5-year axillary recurrence rate (DYE 0.8% vs. DUAL 0.6%, p=0.096), and 5-year disease-free survival (DYE 93.9% vs. DUAL 93.7%, p=0.402) were similar between the two groups.
IntroductionSince its introduction in 1994, sentinel lymph node (SLN) biopsy (SLNB) has replaced axillary lymph node dissection (ALND) in axillary staging surgery for most cases of operable noninflammatory breast cancer [1]. The theory underlying this change is that one or a few lymph nodes receive the first drainage from a tumor site, and when the sentinel node is free of tumor cells, the other nodes are also free. In the pivotal National Surgical Adjuvant Breast and Bowel Project B-32 study, the reported 8-year disease-free survival (DFS) rate was 82.4% in patients who underwent SLN resection plus ALND and 81.5% in patients who underwent SLN resection alone (with ALND alone if the SLNs were positive; p=0.54). The false-negative rate (FNR) for SLNB was 9.8% [2].
Currently available SLN mapping methods include the use of blue dye, radioisotope, or both. The mapping method can affect the identification rate and FNR of SLNB. Although in some studies, the dye method alone achieved comparable performance to the dual-tracer method, a recent meta-analysis showed that the FNR of methylene blue dye alone was 13%, which is notably high according to the Practice Guidelines of the American Society of Breast Surgeons [3]. In one single-institution study, the FNR was much higher in the dye-only group than in the dye and radiolabeled colloid group (21% vs. 2.8%) [4]. A European study also showed that the FNR was significantly higher in the dye-only group than in the dual-tracer group [5,6]. Another meta-analysis reported that the use of blue dye alone was associated with the highest FNR compared with a radioactive tracer alone or a combination of both [7]. Thus, there are concerns about the inferiority of the dye-only technique, and the use of the dual-tracer method is encouraged as the standard procedure to reduce FNR [8]. However, radioisotope mapping exposes physicians and patients to radiation and creates an additional burden in the clinical setting, with a limited window of time for surgery [9]. Nonetheless, there have been no long-term follow-up studies in the literature to compare sentinel node mapping methods.
The purpose of this study was to compare the long-term oncological outcomes between the dye-only and dye and isotope mapping (DUAL) methods for SLNB in patients with early breast cancer. We aimed to investigate the SLN-positive, axillary recurrence, and DFS rates between the groups using either method.
Materials and Methods1. Patient dataThis research was approved by the Institutional Review Board of Seoul National University Hospital (SNUH) (1912-028-1086). This retrospective cohort study extracted data from the SNUH web database using a software (CDW SUPREME), and manual electronic medical chart review was employed for missing data. In total, 10,389 patients who underwent breast cancer surgery between January 2005 and December 2013 were identified. Patients were enrolled in this study based on the date of their primary breast cancer surgery and were considered eligible if they were clinically diagnosed with lymph node–negative breast cancer, underwent SLNB within this period, and did not receive neoadjuvant therapy prior to breast cancer surgery. Male patients and those with metastatic cancer at the time of diagnosis were excluded. Finally, 5,795 patients were included, of whom, dye mapping only method was used for 2,323 (DYE) and dye and isotope method were used for 3,472 patients (DUAL).
2. Sentinel node mapping proceduresA conventional dye injection method was used for SLNB. After general anesthesia, a 1-mL syringe was used for the intradermal injection of indigo carmine dye, 5–15 minutes before the first surgical incision. Usually, two to four spots were injected around the nipple-areolar complex. A standard SLNB technique was performed after the injection. In the DUAL group, Tc-99m antimony trisulfate was injected at least 1 hour before surgery into the periareolar area. A handheld gamma probe (Neoprobe, Devicor Medical Products, Inc., Cincinnati, OH) was used to identify SLNs with hot uptake. The dye component of the DUAL method was identical to that of the DYE group.
3. Statistical analysisThe independent t test and chi-square tests were performed to detect differences between the two groups. For survival analysis, the Kaplan-Meier method and two-sided log rank test were used. All statistical analyses were performed using SPSS software ver. 25 (IBM Corp., Armonk, NY). Propensity score-matching (PSM) analysis was performed using a logistic regression model based on the patient characteristics. Each patient who underwent the DYE method was matched with one patient who underwent the DUAL method during the same period, and the resulting score-matched pairs were analyzed.
Results1. Demographic and characteristicsA flowchart of the patient selection process is shown in Fig. 1. Of the 5,795 patients included in the study, 2,323 were in the DYE group and 3,472 were in the DUAL group (Table 1). Patients in the DYE group were significantly older than those in the DUAL group (51.4 vs. 48.9 years, p < 0.001) (Table 1). The percentages of patients with pathologic T category (pT) and N category (pN) were higher in the DYE group than in the DUAL group (p < 0.001 and p < 0.001, respectively) (Table 1). There was no significant difference in tumor subtype between the two groups (p=0.130) (Table 1). The DYE group was more likely to undergo total mastectomy (as opposed to breast-conserving surgery) than the DUAL group (39.8% vs. 28.7%, p < 0.001) (Table 1).
2. Lymph node–positive rate between the DYE and DUAL groupsThe number of harvested nodes (3.21 in DYE vs. 3.12 in DUAL, p=0.112) was similar between the two groups (Table 1). We calculated the lymph node–positive rate (LNPR) in the two groups, because if FNR of SLNB were different between the groups, LNPR would be different. In the entire sample, a significant difference in LNPR was observed between the DYE and DUAL groups (34.7% vs. 29.6%, p < 0.001) (Table 1). However, there was no significant difference when comparing LNPR by subgroups according to tumor size (Table 2). In addition, in a multivariate logistic regression analysis, the mapping method (DYE vs. DUAL) was not significantly associated with LNPR (p=0.758) (Table 3). We also did PSM between the two groups with matching factors of age and cT category for LNPR comparison. There was no difference in LNPR between the groups after PSM (31.6% in DYE group and 30.6% in DUAL group, p=0.509).
3. PSM and comparison of oncologic outcome in pN0 patients between the DYE and DUAL groupsWe compared the long-term oncological outcome between the DYE and DUAL groups. If the dye-only method was inferior to the dual method (with higher FNR), it could be assumed that the DYE group would have more disease recurrences than the DUAL group among pN0 patients. Thus, only pN0 patients were included for the PSM between the DYE and DUAL groups. Variables, including age, pT cagtegory, surgery (total mastectomy vs. breast-conserving surgery), and adjuvant therapies, were matched between the two groups (Table 4). The final number of patients was 1,441 in each cohort after PSM.
The median follow-up duration was 8.4 years for the DYE group and 8.9 years for the DUAL group. There was no significant difference in the 5-year axillary recurrence rate between the DYE and DUAL groups (0.8% vs. 0.6%, p=0.096) (Fig. 2). In a multivariate analysis using the Cox proportional-hazard model, young age, higher pT category, and estrogen receptor negativity were significantly associated with axillary recurrence, but SLN mapping method (DYE vs. DUAL) was not a significant predictor (Table 5).
DiscussionTo the best of our knowledge, this is the first study to compare dual-agent vs. dye-only mapping for SLNB in terms of long-term oncological outcomes. In our study, there was no significant difference in axilla recurrence rates or DFS between the two groups. We assumed that if the accuracy of the DUAL method was better than that of DYE method with lower FNR, the proportion of patients with tumor-positive lymph nodes would be higher in the DUAL group. However, there were more lymph node-positive patients in the DYE group than in the DUAL group (34.7% vs. 29.6%). This seemed due to the imbalance between the two groups, because the DYE group had a higher proportion of patients in the clinical stage than the DUAL group (Table 1). In subgroup comparisons of LNPR according to tumor size and in a multivariate analysis, LNPRs were not significantly different between the two groups.
SLNB has become an accepted standard procedure for the axilla staging of patients with clinically node-negative early breast cancer. For SLN mapping methods, blue dye alone, radioisotope alone, and use of a dual-tracer are associated with SLN identification rates of 90%, 95%, and 98%, respectively [8]. Motomura et al. [6] showed that a combination of blue dye and radioisotope mapping was superior to the use of dye alone for SLN identification (detection rate 95% vs. 84%, sensitivity 100% for the combination). A systemic review by He et al. [10] reported that the combination of radioisotope and blue dye mapping for SLNB had higher a SLN detection rate than that of radioisotope alone. Other studies have also shown the superiority of the dual-tracer method over single-tracer methods, with higher SLN detection rates and lower FNRs [11,12]. However, there are issues regarding the inconvenience and safety of radioisotope use. Handling radioisotopes requires special training and education; patients require additional injections at least a few hours before surgery, and there is also the risk of potential radiation exposure not only to patients, but also to the surgical staff, staff in the radiology department performing the preoperative imaging and tumor site localization, and pathology staff handling the surgical specimens. Previous studies have shown that the amount of radiation exposure through SLNB is minimal; however, reports on the long-term effects of very frequent exposure to isotopes are rare [13,14]. Therefore, many surgeons and institutions still use the blue dye alone method for SLNB [15].
A possible reason such comparisons of SLN mapping methods are not conducted more often is that surgeons and institutions often have their own preference for SLN mapping methods. Therefore, a single center is unlikely to have data on different methods. At SNUH, we use Tc-99m antimony trisulfate, which is injected a few hours before surgery in the nuclear medicine department. Thus, for early morning surgeries, we cannot use radioisotopes for SLNB due to a lack of time. Differences between the two groups in patient and treatment characteristics were due to this practice. Older women and those requiring total mastectomy were usually scheduled for early morning surgery. Therefore, the dye-only method was more frequently used in these patients.
In almost all cases in this study, we performed frozen biopsy. We performed ALND if the frozen result was positive for tumor in SLNs. Moreover, ALND was performed if SLN was later confirmed to have metastasis in the permanent section. We had no data on sentinel node detection failure rate in both groups.
In this study, the axillary recurrence rate was 0.6%–0.8%, which is equivalent to that of recent clinical trials (0.4%–0.9%) using SLNB for axillary staging [16]. There was no significant difference in the axillary recurrence and DFS rates between the DYE and DUAL groups after PSM, although there was a significant difference before matching. Patients in the DYE group had older patients, higher stage, and underwent total mastectomy more frequently. These imbalances could have negatively affected outcomes in the DYE group, thus necessitating PSM analysis.
There were some limitations to this study. First, this was a retrospective analysis undertaken in a single institution, which made it vulnerable to selection bias. Although we performed PSM analysis to adjust for differences in variables for long-term outcomes, uncontrolled confounding factors seemed unavoidable.
In conclusion, in this single-institution, 9-year follow-up study, the blue dye-only SLN mapping method was not inferior to the dual method (dye and radioisotope) with regard to long-term axillary recurrence and DFS. We believe that, considering the convenience and lower cost of dye-only mapping, this procedure can be safely recommended in patients who do not receive neoadjuvant systemic therapy prior to surgery and are being treated by an experienced surgeon.
NotesEthical Statement This retrospective study, which involved human participants, was conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The research was approved by the SNUH Institutional Review Board with a waiver of informed consent (1912-028-1086). Author Contributions Conceived and designed the analysis: Lim C, Jung JG, Han W. Collected the data: Lim C, Kang E, Jung JG, Cheun JH. Contributed data or analysis tools: Lim C, Lee HB, Moon HG, Han W. Performed the analysis: Lim C, Kang E, Jung JG, Kim HK. Wrote the paper: Lim C, Han W. Funding acquisition: Lee HB, Han W. Supervision: Lim C, Lee HB, Moon HG, Han W. AcknowledgmentsWe thank the Korea Health Technology R&D Project of the Korea Health Industry Development Institute and the Institute for Information and Communications Technology Promotion grant funded by the Korea government (MSIT) for their financial support of this study.
This study was supported by grants from the Korea Health Technology R&D Project of the Korea Health Industry Development Institute (HI18C2282 to WH) and from the Institute for Information and Communications Technology Promotion grant funded by the Korea government (MSIT) (2018-0-00861 to HBL).
Fig. 1Flowchart of the patient selection process. DUAL, dye and isotope mapping; SNUH, Seoul National University Hospital. 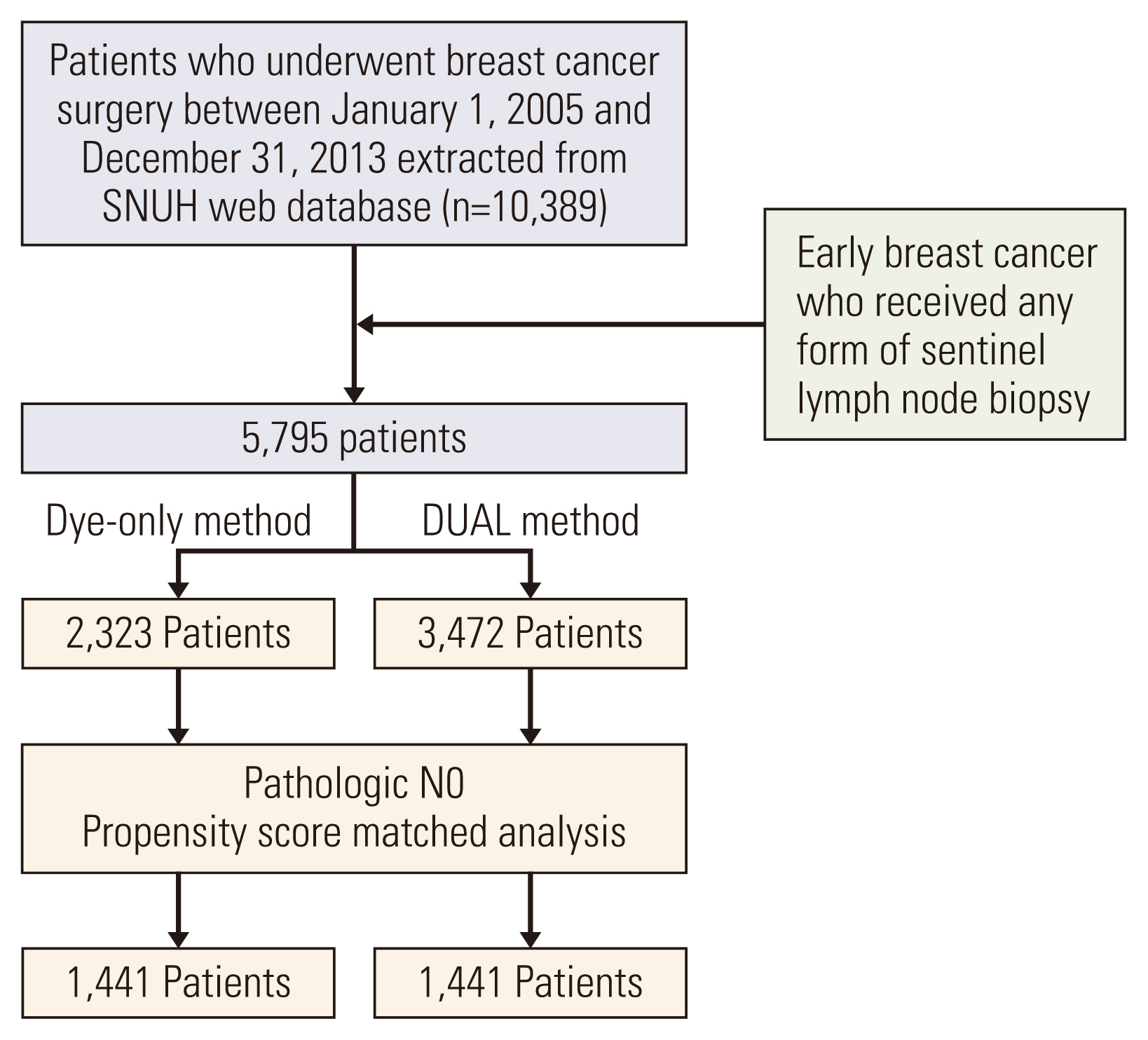
Fig. 2Kaplan-Meier curve for axillary recurrence rate, comparing the dye-only (blue line) and DUAL (red line) groups after propensity score-matched analysis. 5YARR, 5-year axillary recurrence rate; DUAL, dye and isotope mapping. 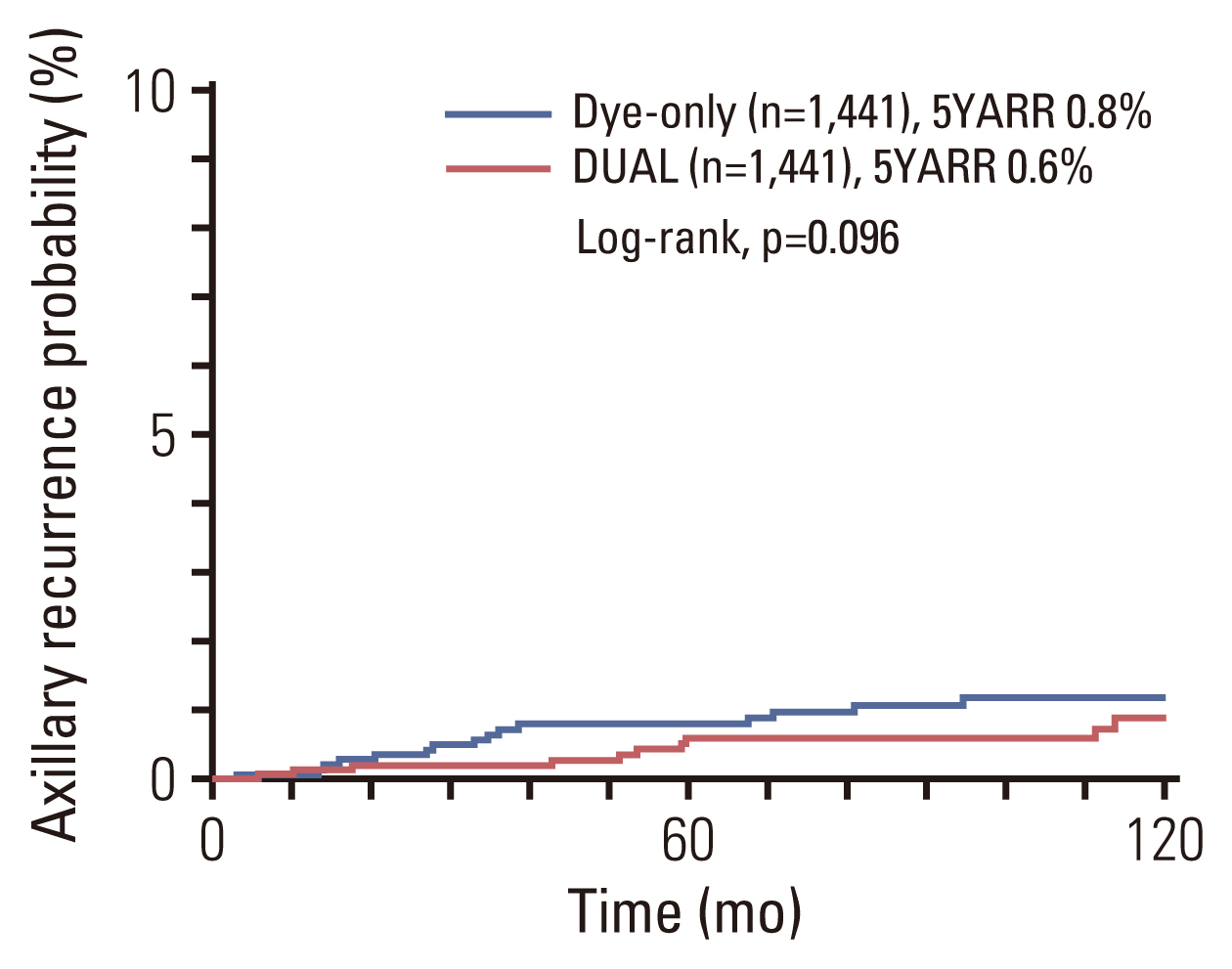
Fig. 3Kaplan-Meier curve for disease-free survival, comparing the dye-only (blue line) and DUAL (red line) groups after propensity score-matched analysis. 5YDFS, 5-year disease free survival rate; DUAL, dye and isotope mapping. 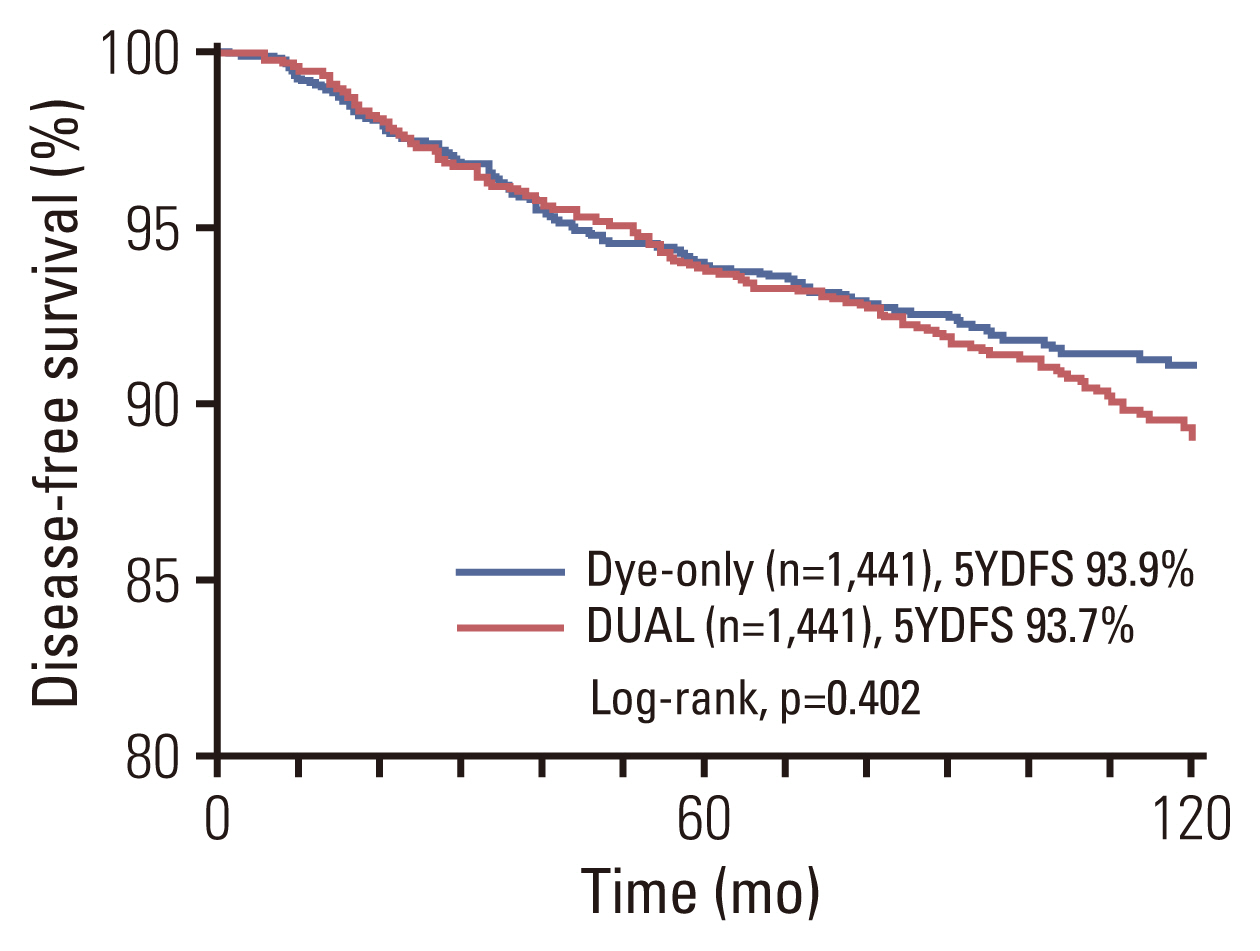
Table 1Demographic and clinical information of the patients enrolled in the study Values are presented as number (%) or mean±standard deviation. ALND, axillary lymph node dissection; BCS, breast-conserving surgery; BMI, body mass index; DUAL, dye and isotope mapping; HER2, human epidermal growth factor receptor 2; LN, lymph node; PR, progesterone receptor; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; TM, total mastectomy; TNBC, triple-negative breast cancer. Table 2Number of lymph node-positive patients according to tumor size Table 3Univariate and multivariate Cox-regression analyses for lymph node–positive rate Table 4Demographic and clinical information of the pathologic N0 patients after propensity score-matched analysis Values are presented as mean±standard deviation or number (%). ALND, axillary lymph node dissection; BCS, breast-conserving surgery; BMI, body mass index; DUAL, dye and isotope mapping; HER2, human epidermal growth factor receptor 2; LN, lymph node; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; TM, total mastectomy; TNBC, triple-negative breast cancer. Table 5Univariate and multivariate Cox-regression analyses for axillary recurrence Table 6Univariate and multivariate Cox-regression analyses for disease-free survival References1. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8.
2. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
3. Li J, Chen X, Qi M, Li Y. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: a systematic review and meta-analysis. PLoS One. 2018;13:e0204364.
4. Syme DB, Collins JP, Mann GB. Comparison of blue dye and isotope with blue dye alone in breast sentinel node biopsy. ANZ J Surg. 2005;75:817–21.
5. Radovanovic Z, Golubovic A, Plzak A, Stojiljkovic B, Radovanovic D. Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur J Surg Oncol. 2004;30:913–7.
6. Motomura K, Inaji H, Komoike Y, Hasegawa Y, Kasugai T, Noguchi S, et al. Combination technique is superior to dye alone in identification of the sentinel node in breast cancer patients. J Surg Oncol. 2001;76:95–9.
7. Pesek S, Ashikaga T, Krag LE, Krag D. The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg. 2012;36:2239–51.
8. Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15:e351–62.
9. Aihara T, Takatsuka Y. Dye-guided sentinel node biopsy revi-sited; validation and observational study from a single institute. Breast Cancer. 2003;10:254–9.
10. He PS, Li F, Li GH, Guo C, Chen TJ. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer. 2016;16:107.
11. Cody HS 3rd, Fey J, Akhurst T, Fazzari M, Mazumdar M, Yeung H, et al. Complementarity of blue dye and isotope in sentinel node localization for breast cancer: univariate and multivariate analysis of 966 procedures. Ann Surg Oncol. 2001;8:13–9.
12. Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20.
13. Gentilini O, Cremonesi M, Trifiro G, Ferrari M, Baio SM, Cara-cciolo M, et al. Safety of sentinel node biopsy in pregnant patients with breast cancer. Ann Oncol. 2004;15:1348–51.
14. Krynyckyi BR, Miner M, Ragonese JM, Firestone M, Kim CK, Machac J. Technical aspects of performing lymphoscintigraphy: optimization of methods used to obtain images. Clin Nucl Med. 2000;25:978–85.
|
|
|||||||||||||||||||||||||||||||||||||||