AbstractPurposeThe advances in the treatment of retinoblastoma have enabled salvaging the globe in advanced stages with intra-arterial chemotherapy (IAC). We developed a strategy of alternate application of systemic intravenous chemotherapy (IVC) and IAC (referred to as alternate systemic IVC and IAC; ASIAC) to reduce central nervous metastases during IAC and examined its efficacy and safety in eye globe salvage in this study.
Materials and MethodsBetween January 2010 and February 2021, 43 eyes of 40 patients received ASIAC treatment for retinoblastoma at the Yonsei Cancer Center, Yonsei University Health System. Their medical records were reviewed retrospectively to evaluate the eye salvage rate (ESR), defined from diagnosis to enucleation. High-risk retinoblastoma was defined as group D or E by the International Classification of Retinoblastoma.
ResultsThe study enrolled 38 and five cases of high-risk and low-risk retinoblastoma, respectively. In total, 178 IAC and 410 IVC courses were administered, with a median of 4 (interquartile range [IQR], 3.0 to 5.0) IAC and 9 (IQR, 6.0 to 11) IVC courses per eye, respectively. The 5-year ESR was 60.4%±8.7% for the whole cohort, 100% for low-risk retinoblastoma, and 53.6%±9.8% for high-risk retinoblastoma. Among those diagnosed since 2015, the 5-year ESR for high-risk retinoblastoma was 63.5%±14.0%. Fifteen eyes underwent enucleation; no viable tumor was found in three enucleated eyes. There were no deaths in this cohort.
IntroductionRetinoblastoma is a rare malignant tumor that occurs during childhood. If the tumor is surgically removed at the proper time point before the occurrence of metastasis, the survival rate of patients with retinoblastoma approaches 99%. Therefore, in developed countries, the primary purpose of treatment is the preservation of the eye globes without compromising the survival rate [1]. External-beam radiotherapy was considered an effective strategy for eye globe salvage; however, it is no longer used due to long-term complications such as secondary malignancies [1,2]. Intravenous systemic chemotherapy (IVC) has been used for eye salvage and achieving long-term survival; however, the eye salvage rate (ESR) has not been satisfactory, especially in patients with advanced retinoblastoma [3,4]. Since its introduction as a promising approach for eye salvage, intra-arterial chemotherapy (IAC) has become a standard treatment strategy [5–7]. The ESR for IAC ranges between 40%–60% [8,9].
High-risk pathologic features found in enucleated eyes indicate the risk of metastases [10]. The high-risk pathologic features include choroidal invasion, retrolaminar invasion and involvement of the resected end of the optic nerve, iris and ciliary body involvement, anterior chamber involvement, and scleral and extrascleral involvement, although the definition of the features is much debated [11,12]. Adjuvant chemotherapy is required in patients with enucleated eyes with high-risk pathologic features [12–14]. Since patients undergoing treatment aimed at eye preservation can have high-risk pathologic features in clinically advanced retinoblastoma, there are concerns regarding the risk of central nervous system metastases with the use of IAC alone [8,15,16]. Therefore, we introduced an alternate approach of using systemic IVC and IAC (ASIAC) to prevent and eradicate micrometastases to the central nervous system and simultaneously achieve satisfactory survival and eye salvage [17,18]. We developed this strategy over a period of ten years and recently published the treatment outcome of advanced retinoblastoma (International Classification of Retinoblastoma [ICRB] group D or E retinoblastomas), focusing on the efficacy of IAC compared with that of the historical treatment group [19]. Herein, we report the results of our follow-up with the ASIAC approach for the primary treatment for retinoblastoma, including all ICRB groups, focusing on its efficacy and safety.
Materials and Methods1. PatientsWe included 40 patients with retinoblastoma who underwent the ASIAC approach as the primary treatment for eye salvage at the Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea, between January 2010 and February 2021. A total of 43 eyes were treated. The demographic data of the patients, including sex, age and symptoms at diagnosis, RB1 gene mutation, staging according to the Reese-Ellsworth (RE) classification, and the ICRB, were collected.
2. TreatmentAll patients received both IAC and IVC. IVC, which consists of vincristine (1.5 mg/m2, day 1), carboplatin (200 mg/m2, days 1–2), etoposide (150 mg/m2, days 1–2), and cyclosporine (12 mg/kg, days 1–2), was the first choice of treatment and was repeated every 3 weeks.
IAC was administered alternatively with IVC (Fig. 1). A typical set of IAC and IVC comprised one course of IVC and IAC, 3 weeks apart, which was repeated for 6–8 cycles. The number of sets was decided according to the tumor response to the treatment (based on the clinical and magnetic resonance imaging [MRI] findings) via a multidisciplinary discussion (MDD) among a pediatric oncologist, ophthalmologist, and an interventional radiologist. Tumor response was observed clinically and documented photographically and/or ultrasonographically. Progressive disease was defined as the enlargement of tumor in tumor base or height, and/or appearance of new lesions, and/or progression of vitreous seeds based on increased number of seeds, conversion from dust to spheres, or the presence of new preretinal tumors [20].
If the treatment response was considered sufficiently complete by the ophthalmologist, IAC was not continued further; only IVC courses were continued until the completion of the 6–8 cycles. After the completion of the ASIAC approach for each patient, they were followed up every 2 to 3 months using ophthalmologic examinations and MRI scans for 2 years. The interval increased based on the examination findings thereafter. Patients younger than 6 months were administered several courses of IVC while awaiting intra-arterial vascular route maturation, followed by the administration of IAC-IVC sets (i.e., bridge ASIAC).
If this primary treatment failed due to refractoriness (defined as an unsatisfactory response by MDD or recurrence after primary treatment completion), the regimen was changed to a salvage regimen composed of vincristine (1.5 mg/m2, day 1), doxorubicin (45 mg/m2, day 1), and cyclophosphamide (500 mg/m2, days 1–3) and/or further IAC courses for eye salvage. We did not perform external-beam radiotherapy to preserve the eye.
Our IAC technique has been described in a previous report [5]. Briefly, the femoral artery was punctured. Subsequently, 4-French angiocatheters were passed through the aorta and carotid artery, and a Marathon flow microcatheter was positioned into the ophthalmic artery with 20–30 minutes of melphalan and/or topotecan infusion. All procedures were performed by a qualified and experienced interventional radiologist at our institution. The doses of melphalan and topotecan were determined according to the patient’s age (melphalan: < 1 year, 3 mg; 1–2 years, 4 mg; > 3 years, 5 mg; topotecan: < 1 year, 0.5 mg; > 1 year, 1.0 mg). We initially used melphalan alone for IAC (through 2014). Melphalan/topotecan was adopted in 2015 after receiving approval from the regulatory government agency of the health insurance (Health Insurance Review and Assessment Service, Republic of Korea) for the use of this regimen for the treatment of retinoblastoma.
IAC-related complications were closely monitored for 1–2 days in the hospital. All treatment-related adverse events were assessed by the Common Terminology Criteria for Adverse Events, ver. 4.0. After enucleation, pathologists examined the enucleated eye and reported the presence of high-risk pathologic features [10,14].
Focal therapies (e.g., thermotherapy, laser photocoagulation, and cryotherapy) were also performed, as needed. The ophthalmologist meticulously examined the eye after every 1–2 ASIAC sets under general anesthesia and determined the need for focal therapy. Additionally, intravitreal chemotherapy (melphalan, topotecan, or methotrexate) injection was started in 2015. Under general anesthesia, intravitreal injection was performed through the pars plana at 2–3 mm from the limbus, depending on the age of the patients. Intra-vitreous melphalan (25–30 μg in 0.04–0.08 mL), topotecan (10–20 μg in 0.04 mL), or methotrexate (400 μg in 0.05 mL) was prepared in the operating room under sterile conditions. Intravitreal injection was administered through the pars plana route with a 30-G needle [19].
3. Statistical analysisData are presented as the median with interquartile range (IQR), number with percentage, or mean±standard deviation. The salvage duration was calculated from the day of diagnosis to the day of enucleation. Relapse events were defined as follows: recurrence after the completion of treatment for the primary aim or an IVC regimen change after the determination of residual tumor as refractory to the primary treatment by MDD, whichever occurred first. The progression events were defined as relapse events or enucleation, whichever occurred first. The 5-year ESR and progression-free salvage rate (PFSR) were analyzed using the Kaplan-Meier method and log-rank test. Since we adopted melphalan and topotecan as the regimen for IAC and intravitreal chemotherapy in 2015, the 5-year ESR was separately analyzed for the cohort of patients diagnosed after 2015.
All statistical analyses were performed using SPSS ver. 23.0 (IBM Corp., Armonk, NY) and R Statistical Software ver. 3.6.3 (Foundation for Statistical Computing, Vienna, Austria). p-values < 0.05 were considered statistically significant.
Results1. Patient demographics and clinical featuresThe patients comprised 22 men and 18 women (median age at diagnosis, 1.57 years [IQR, 0.91 to 2.50]) (Table 1). Eleven patients had bilateral retinoblastomas; three patients had bilateral retinoblastomas and were treated with the ASIAC approach for both eye globes. Eight patients with bilateral retinoblastomas received ASIAC for only one eye globe. No eyes were classified as RE stages I and II or ICRB A. Thirty-two eyes (74.4%) were classified as RE stage V and 26 eyes (60.5%) as ICRB E. Six eyes (14.0%) were low-risk according to the RE stage and five eyes (11.6%) were low-risk according to the ICRB group (Table 2). Other detailed clinical information regarding the treated eye globes is summarized in S1 Table.
2. ASIAC chemotherapy detailsA total of 410 IVC courses (median per eye, 9 [IQR, 6.0 to 11.0]) were administered (S1 Table). Among these, 312 (median per eye, 7 [IQR, 6 to 8]) were performed before progression as a primary salvage attempt. Additionally, 178 IAC courses were administered, with a median of 4 (IQR, 3.0 to 5.0) cycles per eye. Among these, 153 (median per eye, 3 [IQR, 3 to 5]) were administered before relapse. Most patients received < 6 IAC courses (Table 1, Fig. 2).
3. Eye salvage rateOverall, the 3-year and 5-year ESRs were 69.0%±7.5% and 60.4%±8.7%, respectively (Fig. 3A). According to the ICRB group, the 5-year ESR was 100% for groups B and C, 35.8%±19.2% for group D, and 70.4%±9.4% for group E (Fig. 3B). There was late enucleation in the ICRB group B eyes after 7.41 years of follow-up. The 3-year and 5-year ESRs were 100% and 100%, respectively, in the low-risk group, and 64.3%±8.4% and 53.6%±9.8%, respectively, in the high-risk group, as defined by the ICRB (Fig. 3C).
The cohort of patients diagnosed after 2015 showed a tendency towards a better 5-year ESR than those diagnosed before 2015 (67.2%±12.7% vs. 50.0%±12.5%, respectively, p=0.23) (Fig. 3D). In the cohort diagnosed after 2015, the 5-year ESR was 100% in the low-risk group and 63.5%±14.0% in the high-risk group (Fig. 3E). According to the IAC regimen for high-risk retinoblastomas, the melphalan/topotecan group showed a tendency towards a higher 5-year ESR than that in the melphalan-alone group (56.1%±13.3% vs. 40.0%±15.5%, respectively, p=0.17) (Fig. 3F).
4. PFSR and additional treatment detailsTwenty-seven eyes in 21 patients progressed or underwent enucleation (S1 Table). The overall 3-year and 5-year PFSRs were 35.4±7.7% and 31.9%±7.7%, respectively (Fig. 4A). The 5-year PFSR was 28.8%±7.8% in the high-risk group and 60.0%±21.9% in the low-risk group (p=0.18) (Fig. 4B).
After progression, 25 IAC courses (median per eye, 0 [IQR, 0 to 1]) were administered in an attempt to salvage 13 eyes. Twelve eyes with progression were not treated with additional IAC courses to salvage the globes. Additionally, 98 IVC courses (median per eye, 2 [IQR, 0 to 4]) were administered to 23 eyes. Two eyes with progression were not treated with additional IVC courses to salvage the globes. There was no difference in PFSR according to the IAC regimen (melphalan-alone group vs. melphalan/topotecan group, p=0.34, data not shown).
5. Enucleation and surgical pathologyFifteen eyes underwent enucleation (Table 3). Among these, six (40%) had high-risk pathologic features. Three eyes (20%) showed no residual retinoblastoma after enucleation during the pathologic examination. The reasons for enucleation were persistent or recurrent retinoblastoma tumor, including vitreous seeding (n=11), vitreous opacity caused by intravitreous hemorrhage, or retinal detachment rendering ophthalmology examination of the retina impossible (n=3), and atrophy of the eyeball (n=1). There were no deaths.
6. Adverse eventsAll-grade events among 178 procedures were as follows: 14 (7.9%) eyelid swelling events, 11 (6.2%) conjunctival injections, and 11 (6.2%) erythemas. Grade 3 or higher events comprised two cases of oculovagal reflex causing systemic hypotension, clinically identified decreased visual acuity, eyelid swelling, and erythema (Table 4). There were no seizures or cerebrovascular accident events.
DiscussionIAC for retinoblastoma is regarded as the standard treatment approach for salvaging eyeballs and ensuring survival [8,9]. External-beam radiotherapy is no longer accepted as a primary treatment and is now regarded as a treatment failure event similar to enucleation [3]. Further, the ESR is not satisfactory with IVC alone [3]. With IAC, the ESR is markedly increased compared with the historical results; however, concerns remain regarding central venous system recurrences, and consequently, unavoidable mortality due to relapse [8,9].
In patients with clinically advanced retinoblastoma, high-risk pathologic features requiring adjuvant chemotherapy are often observed after enucleation [10,14]. Therefore, there is a risk of occult pathologic high-risk features with IAC alone as there are no reliable detection modalities to confirm a lack of micrometastases in the central nervous system without and before enucleation. In a recently updated systemic review on the efficacy of IAC alone, the ESR was around 60% for low-risk retinoblastoma and 40% for high-risk retinoblastoma. Metastases were found in approximately 2% of patients [8,9].
To overcome this small but evident risk, we used ASIAC, alternating IAC-IVC treatment for high-risk, advanced-stage retinoblastoma to eradicate potential micrometastasis while undergoing eye preservation treatment, since 2010 [17,18]. Recently, several researchers published similar approaches to increase the ESR [21,22]. Using ASIAC, we achieved a 5-year ESR of 100% for low-risk retinoblastoma and > 50% for high-risk retinoblastoma; after 2015, the ESR for high-risk retinoblastoma was > 60%. Furthermore, no deaths occurred due to retinoblastoma, although the study cohort was relatively small.
The ESR of the treatment cohort showed a tendency to increase after 2015. In 2015, we adopted a two-drug regimen of IAC for retinoblastoma as well as intravitreal chemotherapy. These changes in the treatment practices may have increased the ESR. The three-drug regimen is the current standard worldwide and shows excellent ESR [23]. In addition, the role of intravitreal chemotherapy in the increasing rate of salvage of the eye globe is well known [24].
To avoid potential recurrences and relapses, the cases with greatly advanced retinoblastoma, such as RE stage E eyes, are often excluded from an eye salvage approach with IAC alone; primary enucleation is recommended for this group [2,25,26]. However, using ASIAC, we were able to salvage a substantial number of ICRB group D and E eyes without compromising the survival rate.
One shortcoming of ASIAC is the concern regarding the short-term and long-term treatment-related complications. IAC is not without complications, and the optimal combination, number, and intensity of IAC and IVC courses remain uncertain [27]. We initially performed as many as nine IAC courses with combined IVC to salvage the eyeballs. However, we later limited the number of IAC courses to < 6, as much as possible; we also limited the number of IVC courses. Currently, we decide whether to proceed with enucleation or continue with ASIAC under MDD at the time of the third or fourth course to reduce the long-term complications and treatment toxicity. However, the optimal combination of IAC and IVC and the suitable roles of each treatment for eye salvage should be further investigated.
It is difficult to determine the exact timing of enucleation during the course of eyeball salvage in retinoblastoma. Such decisions are dependent on clinical ophthalmology examinations and MRI findings [28,29] and are often determined to be wrong (i.e., the pathology report does not reveal any residual viable tumor in the enucleated eye). The triggers for enucleation include tumor progression based on clinical examination findings, persistent and refractory vitreous seeding nodules, or enhanced ophthalmic nerve on MRI. There have been cases with discrepant clinical findings and pathologic results. Among the clinical findings considered to indicate the risk of impending central nervous system metastases, retinal detachment is under debate. Although retinal detachment can be caused by disease progression, it could also be a complication of IAC or intravitreal chemotherapy [8,27]. It also occurs without any discernible reason. Therefore, when deciding to enucleate an eye with retinoblastoma and retinal detachment, a comprehensive review of the clinical course and MDD are strongly recommended.
One challenging problem in retinoblastoma is the frequent relapse after the planned courses of treatment [30]. Patient treatment courses are highly intricate, rendering management difficult. Thus, it is difficult to directly compare the efficacy of different treatment strategies [31,32]. There have been a substantial number of cases of progression. Accordingly, we had to provide further treatment to consolidate the definitive and permanent control of the residual retinoblastoma. Most progressions were identified before 3 years from diagnosis; therefore, special concerns should be conveyed regarding the progression during the first 3 years of follow-up. Furthermore, the requirement of a sufficiently long period of follow-up to confirm the result of retinoblastoma treatment should be emphasized [15]. Frequent relapses place physical and psychological burdens on patients, families, and physicians, leading to drop-off and treatment give-ups. Therefore, treatment compliance is another key element for success in the long, complex course of eye salvage treatment. If there is a risk of drop-out due to various physio-psycho-social issues, primary enucleation, instead of eye salvage, should be considered and discussed. Measures to enhance treatment compliance also should be provided [33]. Collaboration among the oncologist, ophthalmologist, radiologist, pathologist, patient, and families is strongly needed to complete retinoblastoma treatment successfully [1].
This study has some limitations. Although we used combined intravitreous chemotherapy in patients with vitreous seeding, we could not analyze the role of the intravitreous treatment since the retinoblastoma treatment course was highly complex. Although we adopted a protocol based on systematic treatment in patients with retinoblastoma, the study was not designed as a prospective clinical trial due to the complexity of the management for each patient. Another limitation is the lack of systematic visual function evaluations during the treatment and follow-up courses. Furthermore, the follow-up period was relatively short, and the ESR should be further matured. Although the number of patients in this cohort was small, it was substantial considering the rarity of retinoblastoma in Korea [34].
In conclusion, the ASIAC approach is an effective treatment strategy for increasing the ESR while maintaining satisfactory survival. Issues remain regarding the optimal strategy for combining IAC and IVC, and further investigation should be performed to enhance the efficacy and safety of this approach.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Severance Institutional Review Board (4-2021-0345). The informed consent was waived due to the type of the research of this retrospective study. Author Contributions Conceived and designed the analysis: Han JW, Lee CS, Lee SC, Kim BM, Kim DJ, Lyu CJ. Collected the data: Han JW, Lee CS, Lee SC, Kim BM, Kim DJ, Lyu CJ. Contributed data or analysis tools: Han JW, Lee CS, Hahn SM, Ahn WK, Kim HS, Yun H, Lee SC, Kim BM, Kim DJ, Lyu CJ. Performed the analysis: Han JW, Lee CS, Lee SC, Kim BM, Kim DJ, Lyu CJ. Wrote the paper: Han JW, Lee CS, Kim BM, Kim DJ, Lyu CJ. Fig. 1One cycle of Alternate Systemic and Intraarterial Chemotherapy (ASIAC). One course of ASIAC comprises combination systemic intravenous chemotherapy and intra-arterial chemotherapy (IAC). Each course is delivered to the patient 2–3 weeks apart. A maximum of 6–8 courses are planned for the primary aim of salvaging the eye with the newly developed retinoblastoma. 
Fig. 3Eye salvage rate (ESR). (A) The ESR of the whole cohort is shown. (B) The ESR according to the international classification of retinoblastoma (ICRB) is shown. (C) The ESRs of the low- and high-risk groups, as defined by the ICRB, are shown. (D) The ESR according to the year at diagnosis is shown. Patients diagnosed before and after January 2015 were compared. (E) The ESR of the cohort diagnosed after January 2015. ICRB high-risk vs. low-risk. (F) The ESR of the high-risk group according to the intraarterial chemotherapy regimen (melphalan/topotecan vs. melphalan alone). 
Fig. 4Progression-free salvage rate. (A) The progression-free salvage rate of the whole cohort is shown. (B) The progression-free salvage rates of the high- and low-risk groups, as defined by the international classification of retinoblastoma, are shown. 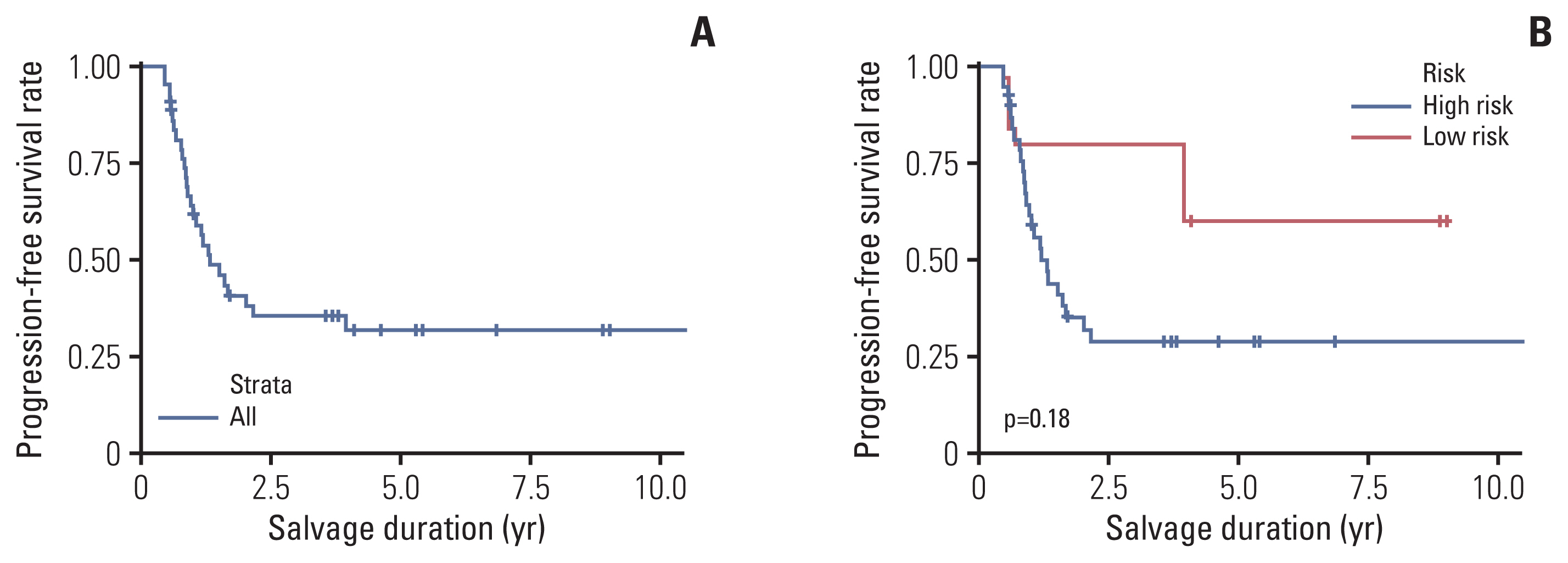
Table 1Patient characteristics and detailed treatment information Table 2Retinoblastoma staging information
Table 3Reasons for nucleation and pathologic findings in the enucleated eyes
a) For the eye globe number, refer to the detailed eye globe information in the S1 Table. Table 4Adverse events during the intra-arterial chemotherapy procedures References1. Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012;379:1436–46.
2. Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015;133:1341–7.
3. Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007;67:2173–85.
4. Kim H, Lee JW, Kang HJ, Park HJ, Kim YY, Shin HY, et al. Clinical results of chemotherapy based treatment in retinoblastoma patients: a single center experience. Cancer Res Treat. 2008;40:164–71.
5. Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129:732–7.
6. Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepa-lli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014;121:1453–60.
7. Suzuki S, Kaneko A. Management of intraocular retinoblastoma and ocular prognosis. Int J Clin Oncol. 2004;9:1–6.
8. Ravindran K, Dalvin LA, Pulido JS, Brinjikji W. Intra-arterial chemotherapy for retinoblastoma: an updated systematic review and meta-analysis. J Neurointerv Surg. 2019;11:1266–72.
9. Yousef YA, Soliman SE, Astudillo PP, Durairaj P, Dimaras H, Chan HS, et al. Intra-arterial chemotherapy for retinoblastoma: a systematic review. JAMA Ophthalmol. 2016;134:584–91.
10. Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996;77:1206–13.
12. Sastre X, Chantada GL, Doz F, Wilson MW, de Davila MT, Rodriguez-Galindo C, et al. Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med. 2009;133:1199–202.
13. Kaliki S, Shields CL, Shah SU, Eagle RC Jr, Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011;129:1422–7.
14. Chevez-Barrios P, Eagle RC Jr, Krailo M, Piao J, Albert DM, Gao Y, et al. Study of unilateral retinoblastoma with and without histopathologic high-risk features and the role of adjuvant chemotherapy: a Children’s Oncology Group study. J Clin Oncol. 2019;37:2883–91.
15. Pekacka A. The role of intraarterial chemotherapy in the management of retinoblastoma. J Ophthalmol. 2020;2020:3638410.
16. Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Chitale R, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr. 2012;10:175–81.
17. Hahn SM, Kim HS, Kim DJ, Lee SC, Lyu CJ, Han JW. Favorable outcome of alternate systemic and intra-arterial chemotherapy for retinoblastoma. Pediatr Hematol Oncol. 2016;33:74–82.
18. Choi S, Han JW, Kim H, Kim BS, Kim DJ, Lee SC, et al. Combined chemotherapy and intra-arterial chemotherapy of retinoblastoma. Korean J Pediatr. 2013;56:254–9.
19. Lee DH, Han JW, Hahn SM, Kim BM, Lyu CJ, Lee SC, et al. Changes in treatment patterns and globe salvage rate of advanced retinoblastoma in Korea: efficacy of intra-arterial chemotherapy. J Clin Med. 2021;10:5421.
20. Berry JL, Munier FL, Gallie BL, Polski A, Shah S, Shields CL, et al. Response criteria for intraocular retinoblastoma: RB-RECIST. Pediatr Blood Cancer. 2021;68:e28964.
21. Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Xia J, et al. Evaluating primary intra-arterial chemotherapy versus intravenous plus intra-arterial chemotherapy for advanced intraocular retinoblastoma. Cancer Chemother Pharmacol. 2020;85:723–30.
22. Xu K, Liu J, Zhang C. Intra-arterial chemotherapy combined with VEC intravenous chemotherapy in the treatment of advanced retinoblastoma. J BUON. 2020;25:1199–205.
23. Marr BP, Brodie SE, Dunkel IJ, Gobin YP, Abramson DH. Three-drug intra-arterial chemotherapy using simultaneous carboplatin, topotecan and melphalan for intraocular retinoblastoma: preliminary results. Br J Ophthalmol. 2012;96:1300–3.
24. Shields CL, Alset AE, Say EA, Caywood E, Jabbour P, Shields JA. Retinoblastoma control with primary intra-arterial chemotherapy: outcomes before and during the intravitreal chemotherapy era. J Pediatr Ophthalmol Strabismus. 2016;53:275–84.
25. Grigorovski N, Lucena E, Mattosinho C, Parareda A, Ferman S, Catala J, et al. Use of intra-arterial chemotherapy for retinoblastoma: results of a survey. Int J Ophthalmol. 2014;7:726–30.
26. Scelfo C, Francis JH, Khetan V, Jenkins T, Marr B, Abramson DH, et al. An international survey of classification and treatment choices for group D retinoblastoma. Int J Ophthalmol. 2017;10:961–7.
27. Monroy JE, Orbach DB, VanderVeen D. Complications of intra-arterial chemotherapy for retinoblastoma. Semin Ophthalmol. 2014;29:429–33.
28. de Graaf P, Goricke S, Rodjan F, Galluzzi P, Maeder P, Castelijns JA, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol. 2012;42:2–14.
29. Shields CL, Palamar M, Sharma P, Ramasubramanian A, Leahey A, Meadows AT, et al. Retinoblastoma regression patterns following chemoreduction and adjuvant therapy in 557 tumors. Arch Ophthalmol. 2009;127:282–90.
30. Friedman DL, Krailo M, Villaluna D, Gombos D, Langholz B, Jubran R, et al. Systemic neoadjuvant chemotherapy for Group B intraocular retinoblastoma (ARET0331): a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017;64:e26394.
31. Ghassemi F, Rahmanikhah E, Roohipoor R, Karkhaneh R, Faegh A. Regression patterns in treated retinoblastoma with chemotherapy plus focal adjuvant therapy. Pediatr Blood Cancer. 2013;60:599–604.
32. Francis JH, Levin AM, Zabor EC, Gobin YP, Abramson DH. Ten-year experience with ophthalmic artery chemosurgery: ocular and recurrence-free survival. PLoS One. 2018;13:e0197081.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||