AbstractPurposeThe aim of this study is to evaluate the safety and efficacy of ex vivo activated and expanded natural killer (NK) cell therapy (SNK01) plus pembrolizumab in a randomized phase I/IIa clinical trial.
Materials and MethodsOverall, 18 patients with advanced non–small cell lung cancer (NSCLC) and a programmed death ligand 1 tumor proportion score of 1% or greater who had a history of failed frontline platinum-based therapy were randomized (2:1) to receive pembrolizumab every 3 weeks +/− 6 weekly infusions of SNK01 at either 2×109 or 4×109 cells per infusion (pembrolizumab monotherapy vs. SNK01 combination). The primary endpoint was safety, whereas the secondary endpoints were the objective response rate (ORR), progression-free survival (PFS), overall survival, and quality of life.
ResultsSince no dose-limiting toxicity was observed, the maximum tolerated dose was determined as SNK01 4×109 cells/dose. The safety data did not show any new safety signals when SNK01 was combined with pembrolizumab. The ORR and the 1-year survival rate in the NK combination group were higher than those in patients who underwent pembrolizumab monotherapy (ORR, 41.7% vs. 0%; 1-year survival rate, 66.7% vs. 50.0%). Furthermore, the median PFS was higher in the SNK01 combination group (6.2 months vs. 1.6 months, p=0.001).
IntroductionThe incidence of non–small cell lung cancer (NSCLC), consisting approximately 80% of lung cancers, has drastically increased, and NSCLC remains to be one of the leading causes of cancer-related death worldwide [1]. Although platinum-based chemotherapies, such as cisplatin with gemcitabine (GP therapy) or pemetrexed (PP therapy), have been used as the first-line treatment for NSCLC patients, the clinical benefits from these therapies are restricted to only a small portion of patients accompanied with a plateau [1–3]. Recently, the new development of immune checkpoint inhibitors (ICIs) has moved into the breakthrough advances in NSCLC treatment [4]. Pembrolizumab has replaced chemotherapy as the first-line treatment for patients with a programmed death-ligand 1 (PD-L1) tumor proportion score (TPS) of at least 50% [5,6]. However, the low response rates of ICIs for NSCLC patients is still a problem encountered in current immunotherapies.
Many studies aim to improve the efficacy of ICIs. These studies were focused on searching predictive biomarkers to tumor response and novel combination approaches for ICIs. Among the most widely known predictive biomarkers for ICIs are PD-L1, microsatellite instability/defective mismatch repair (MSI/dMMR), and tumor mutational burden [7,8]. Although MSI/dMMR is approved for clinical use in all types of solid tumors and PD-L1 is approved only for clinical use in specific cancer types (e.g., for predicting the response to first-line pembrolizumab monotherapy in NSCLC) [9,10], many researchers suggest that any single biomarker cannot effectively identify the benefit populations. Thus, they think that the specificity and efficacy of prediction will be greatly improved through the combination of multiple factors. Aside from the predictive biomarkers, many studies have been using combinatorial approaches to improve the efficacy of ICIs. Indeed, chemotherapy, radiation therapy, molecularly targeted therapy, and cell therapy are considered as combination regimens [11,12]. However, an effective way to incorporate the molecularly targeted and immune targeted therapies into combination regimens is yet to be determined. Moreover, many problems, including side effects and efficacy, must be taken into consideration when designing the ICI-based combination regimens because the other types of therapy may have significant influence on host immunity or tumor microenvironment.
Natural killer (NK) cells, which are innate lymphocytes, account for 5%–15% of human peripheral blood leukocytes and are considered as a major type of immune cells that can kill foreign target cells [13,14]. NK cells are also an essential population for tumor immunosurveillance by orchestrating the innate immunity in the heterogeneous microenvironment [15,16]. NK cells participate in the immune response against solid and hematopoietic cancer cells by their capacity to recognize the molecular patterns characteristic of stressed cells. Indeed, higher cancer susceptibility and tumor progression to metastasis are significantly associated in NSCLC patients with a higher NK cell count [13,17]. Moreover, unlike T cells, the NK cells can recognize and attack cancer cells without neo-antigen in high mutation loaded patients and loss of MHC expression which often occurs in human cancer [16,18,19]. The NK cells are activated by ligands that are often upregulated in the condition with oncogenic stress [16]. Therefore, the development of NK cell-mediated immunotherapies would be an ideal strategy to increase the efficacy of current T cell–mediated immunotherapy and increase the response rate of current T cell–mediated immunotherapy.
In this study, we generated the non-genetically modified and autologous super NK cells (SNK01) by using the NK cell activation condition. We also investigated the safety and tolerability as well as the preliminary antitumor activity of SNK01 when administered in combination with pembrolizumab in patients with NSCLC.
Materials and Methods1. Study designThe aim of this randomized, open-label, single-center study is to evaluate the safety, tolerability, and anti tumor activity of SNK01 in combination with pembrolizumab in patients with advanced or metastatic NSCLC (PD-L1 TPS ≥ 1%) who had a history of failed frontline platinum-based therapy. The primary endpoint is safety, and the secondary endpoint is efficacy, represented by objective response rate (ORR), progression-free survival (PFS), overall survival, time to progression, and quality of life (QoL).
2. PatientsEligible patients were recruited at Asan Medical Center (Seoul, South Korea) between February 2019 and March 2020. In the phase I study, patients with advanced and/or metastatic NSCLC were sequentially enrolled in cohorts of 3–6 subjects. The eligible subjects received study drugs that begin on cycle 1 day 1 and continued in 3-week cycles until the occurrence of the unequivocal radiographic disease progression using Response Evaluation Criteria in Solid Tumor (RECIST) ver. 1.1 as assessed by the investigator, unacceptable toxicity, or other reasons for discontinuation.
Eighteen patients with advanced NSCLC with a PD-L1+ TPS of 1% or greater who had a history of failed frontline platinum-based therapy were randomized (2:1) to pembrolizumab every 3 weeks +/− 6 weekly infusions of SNK01 at either 2×109 or 4×109 cells per infusion (pembrolizumab monotherapy [cohort 0] vs. SNK combination [cohort 1, 2, respectively]).
3. NK cell isolation and expansionAll the manufacturing and testing procedures used to produce ex vivo expanded NK cells (SNK01) were performed under good manufacturing practice conditions (NKMAX Co., Ltd., Seongnam, Korea). Peripheral blood mononuclear cells (PBMCs) were collected from the leukapheresis products of enrolled patients in the treatment group and then used for NK cell expansions as described previously with some modification [20]. The detailed method for NK cell expansion is described in the Supplementary Methods.
4. Characterization of the NK cellsThe phenotype of culture-expanded NK cells was determined via flow cytometric analysis. For assessing the NK cell activity, cytotoxicity and degranulation assays were performed. The detailed method of these assays is described in the Supplementary Methods.
5. TreatmentsDose escalation was evaluated in a phase I study of SNK01, which was administered in combination with pembrolizumab. The purpose of the dose escalation phase was to gather preliminary safety and tolerability data for SNK01 in combination with pembrolizumab, as well as SNK01 in combination with pembrolizumab, to determine the maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D) for each combination regimen for the phase IIa portion of the study.
The dose escalation followed the standard oncology phase I “3+3” dose escalation design in cohort 1 and cohort 2 (Fig. 1). After cohort 1, The patients were randomly allocated at 1:1 ratio to receive pembrolizumab only (200 mg every 3 weeks) or pembrolizumab in combination with either 2×109or 4×109 cells/dose of SNK01 (weekly infusion for 6 weeks). cohort 0 served as the control group for cohort 1 and cohort 2. Three eligible subjects were initially enrolled into cohort 1. The subjects were administered with 2.0×109 SNK01 in combination with pembrolizumab. If no dose-limiting toxicities (DLTs) were observed during the DLT observation period, three eligible subjects were enrolled into cohort 0 and cohort 2 and received pembrolizumab alone or 4.0×109 SNK01 in combination with pembrolizumab, respectively. Again, if no DLTs occur during the DLT observation period, three more eligible subjects were enrolled into cohort 2 to confirm the MTD/RP2D for SNK01 in combination with pembrolizumab. Following the completion of the DLT observation period in cohorts 1 and 3, additional subjects were allowed.
The expansion of up to three subjects was allowed if 0 of three subjects has a DLT to further examine the preliminary efficacy, while assessing the RP2D, which is consistent with backfilling a cohort. If one subject develops a DLT at a specific dose during the DLT observation period, additional three subjects are enrolled into that same dose cohort. The development of DLTs in more than one of six subjects in a specific dose cohort suggests that the MTD has been exceeded and further dose escalation was not pursued.
A DLT was defined as a Common Terminology Criteria for Adverse Events (CTCAE) grade of ≥ 3 for any adverse event related or at least possibly related to the administration of SNK01 occurring within the DLT observation period. The subjects were eligible for DLT evaluation if they experience a DLT after at least one dose of study drug or do not experience a DLT having taken a minimum of 75% of the doses expected during the DLT observation period. The subjects who did not fulfill these requirements and who discontinued their study participation prior to completing the DLT observation period were replaced for DLT evaluation but remained in the overall safety and efficacy analyses.
6. Follow-up and adverse eventsOn-study imaging for tumor assessments was performed with the use of RECIST ver. 1.1, every 6 weeks (±7 days) after the first dose of the study treatment and should follow calendar days and not be adjusted for delays in cycle starts. The same imaging technique should be used in a subject throughout the study. Safety was monitored via laboratory assessments, physical examinations, and vital signs. It was graded by physicians in accordance with the U.S. National Cancer Institute’s (NCI) CTCAE ver. 5.0.
Subjects who discontinued the study treatment for a reason other than disease progression moved into the long-term follow-up phase and should be assessed every 6 weeks (±7 days) via radiologic imaging to monitor the disease status. Every effort should be made to collect information with regard to the disease status until the start of a new therapy, during a disease progression, at death, or until the end of the study. If a subject prematurely withdraws from the study, all evaluations described under the End of Study Visit were performed. Additionally, once a subject has presented with a confirmed disease progression or starts a new anticancer therapy, the subject moved into the survival follow-up phase and should be contacted through telephone or clinical visit every 12 weeks to assess for survival status until death, withdrawal of consent, or the end of the study, whichever occurs first.
7. Patient-reported outcomesThe patient-reported outcomes (PROs) were collected to evaluate the disease-related symptoms and health-related quality of life (HRQoL) to support the finding of a survival benefit. Moreover, the PROs were collected upon screening: 3rd visit (day of the 2nd pembrolizumab administration), 6th visit (day of the 3rd pembrolizumab administration), 9th to 13th visit (day of the 4th, 6th, 8th, 12th, and 16th pembrolizumab administration), and 14th visit (end-of-treatment visit) for patients who completed one baseline and one post-baseline PRO assessment. The European Organization for the Research and Treatment of Cancer (EORTC) QoL questionnaire and lung cancer module was used to assess the PROs. The PROs reflecting the lung cancer symptoms, commonly reported treatment-related symptoms, functioning in daily life, and HRQoL were collected using two self-administered questionnaires that have been routinely used in lung cancer studies: the EORTC quality-of-life questionnaire (QLQ-C30) and its lung cancer module (QLQ-LC13).
8. Statistical analysisDescriptive statistics were used for the baseline characteristics of the patients. Pearson’s chi-square test and Fisher exact test were used for data comparison, and the Mann-Whitney U test for the comparison of the nonparametric variables. The survival was estimated using the Kaplan-Meier method, and the log-rank test was used to determine the significance of any differences in the survival curves. All tests were two-sided, and a p-value of < 0.05 was considered statistically significant. The SPSS ver. 25.0 (IBM Corp., Armonk, NY) and SAS ver. 9.4 (SAS Institute Inc., Cary, NC) were used for the analyses.
Results1. Characteristics of the NK cell productsTo manufacture the ex vivo expanded NK cell products, the CD56+ cells were isolated from the patients’ PBMCs and expanded as described previously [20]. In freshly isolated CD56+ cells from the leukapheresis products of enrolled patients in the treatment group, the proportion of NK cells (CD56+CD3−) varied among donors (66.47%±18.67%). However, as stated previously [20], after 17–18 days of culture with γ-irradiated KL-1 and LCL feeders in the presence of interleukin (IL)-2 and IL-21, the proportion of NK cells (CD56+CD3−) was significantly increased (99.10%±0.87%) in the NK cell products from all donors with a minimal contamination of the CD3+ T cells (0.76%±0.83%), CD20+ B cells (0.17%±0.14%), and CD14+ monocytes (0.11%±0.13%) (Fig. 2A, S1 Table). In the expansion culture, the NK cells were efficiently expanded (2,858±1,774-fold; median, 1,964; range, 1,171 to 5,867) with high viability (97%±0.94%) (S1 Table), which were sufficient for multiple injections in all donors. As the cytotoxicity of the NK cells is finely regulated by the net balance of signals from activating and inhibitory receptors on their surface, the expression levels of activating and inhibitory receptors were analyzed. The culture-expanded NK cells from all donors highly expressed activating receptors, including NKG2D (98.87%±2.43%), CD16 (87.37%±3.56%), DNAM-1 (98.63%±1.84%), NKp30 (96.05%±5.05%), NKp46 (91.59%±9.01%), inhibitory receptor NKG2A (78.01%±10.60%), and chemokine receptor CXCR3 (95.65%±4.12%), whereas the expression level of inhibitory receptors, CD158a (KIR2DL1; 12.53%±8.36%), CD158b (KIR2DL2/L3; 21.63%±10.08%), and CD158e (KIR2DL1; 12.23%±5.35%), was relatively low. Moreover, the culture-expanded NK cells highly expressed cytotoxic granules of perforin (99.44%±0.62%) and granzyme B (99.47%±0.61%) in all NK cells (Fig. 2B, S2 Table). The cytotoxic activity of culture-expanded NK cells was examined 1 day before injection day (16–17 days of culture) against the standard K562 cells which are a NK-sensitive target and the NCI-H2087 lung adenocarcinoma cells. As expected from high expression levels of several activating receptors and cytotoxic granules, the expanded NK cells from all patients exerted a strong cytotoxic activity against both K562 and NCI-H2087 cells even at a low E:T ratio of 0.5:1 (54.2%±7.9% and 42.2%±5.9% of the K562 and NCI-H2087 targets, respectively) (Fig. 2C, S3 Table). In untreated NK cells (0.74%±0.28%), NK cell degranulation activity was upregulated when cocultured with K562 cells (38.51%±12.76%) or treated with phorbol 12-myristate 13-acetate/ionomycin (88.96%±6.12%) (Fig. 2D, S4 Table). Collectively, we could produce a large number of clinical-grade NK cells (SNK01) with minimal contamination of other immune cells and high cytotoxic activity against cancer cells for multiple injections via ex vivo expansion using two feeder cells and cytokines.
2. Patients and treatmentsBetween February 2019 and March 2020, a total of 20 patients (13 male and 7 female) were enrolled. Table 1 summarizes the baseline characteristics and NK cell activities. The median age was 61 years (range, 31 to 77 years), and the most common histologic type of tumor was adenocarcinoma, except for one pleomorphic carcinoma. The baseline characteristics including the PD-L1 expression status were balanced between the two groups.
As shown in Fig. 1, a total of 18 patients were scheduled to be enrolled and to be randomized for pembrolizumab monotherapy group (6 patients) or pembrolizumab plus SNK01 (SNK combination) group (12 patients). However, two patients in the SNK combination group (one patient in cohort 1 and the other in cohort 2) received a single dose of pembrolizumab and then were dropped out due to serious adverse event (SAE) before initiating SNK01 administration. Therefore, two additional patients were enrolled to the SNK combination group. Thus, the final number of enrolled patients in the study was 20: six were assigned to receive pembrolizumab monotherapy, and 14 to receive SNK combination. Every six patients completed therapy with pembrolizumab alone, pembrolizumab plus 2×109 SNK01, or pembrolizumab plus 4×109 SNK01.
3. MTD determinationNine patients were involved in the dose escalation part of the study and received pembrolizumab plus SNK01. SNK01 was administered intravenously for 6 consecutive weeks (2×109 cells/dose, n=3; 4×109 cells/dose, n=6), except for three patients who were administered with five doses of SNK01 due to a progressive disease. Because no DLT was observed, MTD was determined as SNK01 4×109 cells/dose.
4. SafetyTwenty patients were included for safety analysis. The treatment was well tolerated throughout the trial. Moreover, no adverse events related to SNK01, as well as any new safety signals in the SNK combination group, were observed.
Table 2 summarizes the adverse events. Immune-related hyperthyroidism (n=3), hypothyroidism (n=3), and pneumonitis occurred in the SNK combination group. No grade 3–5 immune-related adverse events (AEs) were observed. The median time to the occurrence of immune-related AEs was 2.7 months after the first pembrolizumab administration (range, 0.7 to 7.4). One immune-related AE occurred before the SNK01 administration due to pembrolizumab, and the other six occurred median 2.2 months after the first SNK01 administration (range, 0.2 to 6.7). The patients receiving pembrolizumab plus SNK01 tended to experience more immune-related AEs than those receiving pembrolizumab alone (35.7% vs. 0%, p=0.26), but it was not statistically significant.
The safety data analyzed by putting the two patients who discontinued the scheduled treatment before the initiation of SNK01 due to SAEs in the pembrolizumab monotherapy group (n=8 for pembrolizumab monotherapy, and n=12 for SNK combination) were similar to that with intention to treat (ITT) population (S5 Table).
5. EfficacyEighteen patients were included for the analysis of efficacy outcomes per protocol, excluding two patients who discontinued the scheduled treatment before the initiation of SNK01 due to SAEs. Table 3 shows the ORR, median PFS, and 1-year survival rate evaluated in the pembrolizumab monotherapy and SNK combination groups. The ORR for the total population was 27.8% (5/18), whereas the ORR for the SNK combination group (41.7%) was superior to that for the pembrolizumab monotherapy group (0%), but the differences were not statistically significant (p=0.11).
At the time of the data cutoff, 14 of 18 patients presented with disease progression, including eight of 12 patients (66.7%) who underwent SNK combination treatment and six of six patients (100.0%) who underwent pembrolizumab monotherapy. Fig. 3A shows the PFS curve in each cohort. The median PFS was significantly longer in patients who underwent SNK combination treatment than in those who underwent pembrolizumab monotherapy (6.2 months vs. 1.6 months, p=0.001, with a median follow-up duration of 17.5 months) (Table 3, Fig. 3B). Seven patients died, including four of 12 patients (33.3%) who underwent SNK combination treatment and three of six patients (50.0%) who underwent pembrolizumab monotherapy. One-year survival rate tended to be higher for the SNK combination treatment than the pembrolizumab monotherapy (66.7% vs. 50.0%, p=0.39). The result of analyses with ITT population (n=6 for pembrolizumab monotherapy, and n=14 for SNK combination) were similar to that with per protocol population (data not shown).
The efficacy outcomes tended to be higher for patients administered with 4×109 cells/dose of SNK01 than those administered with 2×109 cells/dose of SNK01, but the differences were not statistically significant (ORR: 33.3% vs. 50.0%, p=0.19; median PFS: 9.4 vs. 4.8 months, p=0.45; 1-year survival rate: 50.5% vs. 83.3%, p=0.19).
The ORR, PFS, and 1-year survival rate were significantly higher in the patients with the immune-related AE compared to those without AE (ORR: 80.0% vs. 7.7%, p=0.008; median PFS: not reached vs. 1.7 months, p=0.005; 1-year survival rate: 100% vs. 44.9%, p < 0.001).
6. Patient-reported outcomesThe baseline PRO scores during the screening period were similar between the pembrolizumab monotherapy group and SNK01 combination group for all PRO scales. The patients in both groups (pembrolizumab monotherapy vs. SNK01 combination) reported a moderate-to-high HRQoL at baseline. No statistically significant difference was observed in HRQoL between patients receiving pembrolizumab alone and those receiving pembrolizumab plus SNK01 (p=0.15). The SNK combination group showed a longer time to deterioration in physical function and role function than the pembrolizumab monotherapy group did (physical function: hazard ratio [HR], 0.29, p=0.058; role function: HR, 0.18, p=0.036).
The analyses of the mean change from baseline throughout each visit showed a modest trend favorably toward the SNK01 combination group relative to pembrolizumab monotherapy in HRQoL, physical function, role function, and dyspnea and chest pain symptom scales, which was represented by higher values of mean change in functional scales and lower values in symptom scales.
DiscussionICI monotherapy, including pembrolizumab (KEYTRUDA, Merck), has been Food and Drug Administration–approved as the first-line treatment of choice for NSCLC with a PD-L1 TPS of 50% or greater in patients who are not eligible for or who have failed tyrosine-kinase inhibitor treatment [21]. However, restricted populations with NSCLC have a PD-L1 TPS of over 50%, and the clinical benefits of pembrolizumab monotherapy are limited to only a small proportion of NSCLC patients [22]. The median PFS of pembrolizumab monotherapy in previously treated and PD-L1–positive NSCLC patients is known as approximately 5.0 months with the percentage of grade 3–5 treatment-related adverse events being 13% [23]. Recently, to overcome these limitations of current immunotherapies, the combinations of various other therapies with ICIs have been intensively investigated [22,24]. In this study, we evaluated the clinical safety and efficacy of the autologous NK cell and pembrolizumab combination and also investigated the role of immune cells in this combination therapy with both enrolled patients and mouse model.
We observed that T cells as well as NK cells are also participated in the therapeutic process of programmed death-1 (PD-1)/PD-L1 axis blockade, as shown by our xenograft tumors which derived from NSCLC cells depleting T cells, NK cells, and both cells, respectively (S6 Fig.), consistent with previous study [16], indicating that NK cells may play roles in helping to recruit a T cell response and/or by killing tumor cells directly. Given our in vivo immune cell depletion experiment results showing the participation of the NK cells in the therapeutic effects of the PD-1 blockade and the cytotoxic effects of the NK cells on MHC- and neoantigen-deficient cancer cells [14,25], we determined the therapeutic effects of the combination therapy of NK cell and PD-1 blockade for treating NSCLC. In this study, to optimize the cytotoxic ability of autologous NK cells, we established the super NK cells (SNK01) modulating the culture conditions, which resulted in the activation of the NK cells. We also showed an enhanced cytotoxic ability of SNK01 compared with their corresponding NK cells. PD-1 blockade therapy with SNK01 resulted in the enhanced tumor growth inhibition of xenografted tumors by using the NSCLC cells, regardless of the genetically modified PD-L1 overexpression or knockout (S7 Fig.), suggesting that the combination therapy would be also effective in PD-L1–negative NSCLC patients. Moreover, further confirmation of the PD-L1 independency in therapeutic effects of these combinations would widen the clinical benefits of NSCLC patients, avoiding the unnecessary restriction of the patient cohorts.
Next, we aimed to investigate that the clinical usage of pembrolizumab treatment with autologous SNK cells and evaluate the possible usage of combination therapy for NSCLC as the therapy of choice after platinum-based therapy. Our clinical trial data showed that the ORR and PFS were higher in the SNK01 and pembrolizumab combination group (ORR, 41.7%; PFS, 6.2 months) compared with pembrolizumab alone (ORR, 0%; PFS, 1.6 months). The therapeutic effects of combination therapy were also superior to those of pembrolizumab monotherapy (PFS; 5.0 months) from previous KEYNOTE-010 trial [23]. Moreover, four patients in the SNK01 combination group have not presented with disease progression and been continuing with the treatment. Although the baseline characteristics including epidermal growth factor receptor (EGFR) mutational status and the number of previous lines of chemotherapy were not balanced, these results suggest that the SNK01 and pembrolizumab combination treatment has potential to exhibit more clinical benefit for treating NSCLC patients without severe AEs.
A recent study has shown that the combination therapy of allogenic NK cells and pembrolizumab have survival benefits in PD-L1+, advanced NSCLC patients. We also showed the clinical efficacy of combination treatment with autologous NK cells and pembrolizumab in NSCLC patients for the first time. Autologous NK cell infusion alone has shown limited efficacy, possibly because inhibitory receptors on autologous NK cells matches self MHC I presented on tumor cells and this ‘self’ recognition signals subsequently inhibit activation of NK cells, and autologous NK cells derived from cancer patients are actually in immune-suppression state with impaired functions, making these cells difficult to exhibit antitumor capability [26–28]. However, in the present study, autologous NK cell infusion in combination with pembrolizumab showed potential to improve clinical efficacy of pembrolizumab monotherapy. This could be explained by several reasons that pembrolizumab augments NK cells by expressing PD-L1, or interferon-γ secreted by NK cells expresses PD-L1 in cancer cells [29]. Furthermore, autologous NK cell infusion has an advantage of no need to find donors with human leukocyte antigen typing, compared to allogeneic infusion.
From the viewpoint of safety, patients receiving SNK combination experienced more immune-related AEs than those who receiving pembrolizumab monotherapy (35.7% vs. 0%, p=0.26); however, the difference was not statistically significant. There is a possibility that such a numeric difference occurred due to the small number of study patients. In addition, the SNK combination group had a longer PFS than the pembrolizumab monotherapy group, therefore more pembrolizumab was administered (mean, 5.4 times vs 3.5 times; p=0.12), and the follow-up period was also longer (median, 14.6 months vs. 11.0 months; p=0.24). Of note, unlike cyto-toxic chemotherapy, immune-related AEs are known to occur mainly several months after beginning pembrolizumab treatment [30]. Thus, they may explain why immune-related AEs tended to be detected more frequently in SNK combination group. In addition, there are reports that the occurrence of immune-related AEs is a predictor of the efficacy of immunotherapy [31]. Since the response rate tended to be higher in SNK combination group in the present study, it is also possible that more frequent immune-related AEs in SNK combination group was associated with the relatively favorable response. Moreover, in this study, the efficacy outcomes including ORR, PFS, and 1-year survival rate were significantly higher in the patients with the immune-related AE compared to those without AE. Whether SNK01 combination shows better efficacy than immunotherapy alone without increasing immune-related AEs need be confirmed through additional large-scale studies. Until now, there has been no clear discussion on how to prevent immune-related AEs, but close monitoring of the occurrence of the AEs and active and personalized managements for them are necessary [32].
Herein, we conducted phase 1 to evaluate toxicity and a pilot phase 2a study to determine the appropriate dose for future studies. It was difficult to determine the therapeutic dose of SNK01 in a preclinical model because there was no animal model unlike other anticancer drugs. Moreover, for cell therapy, the dose is not always proportional to the toxic/therapeutic effect, and DLT does not easily occur [33]. In the case of autologous NK cells, few adverse events were reported in previous clinical studies since the patient’s own cells were proliferated [34]. In the present study design, it was planned to increase the SNK01 dose up to 4×109 cells/dose, because DLT was less likely to be observed even if the dose was continuously increased. In addition, continuously increasing the number of cells had various limitations such as cell production capacity and the time required for administration. According to the protocol, the maximum dose administered during clinical trials, 4×109 cells/dose, was determined as the MTD.
There are several limitations in this study. The first limitatin is the small number of patients. This study was a phase I/IIa clinical trial of the new treatment option, which will require a further large-scale randomized study based on these results. The second limitation was that the baseline tumor characteristics including EGFR mutation status and the number of previous lines of chemotherapy were not balanced between SNK01 combination and pembrolizumab monotherapy group because of the small number of patients. ORR of pembrolizumab alone was 0%, less than 18% of the previous study [23]. Considering that EGFR-positive patients have shown a poor response to ICI in the previous study [35], the higher EGFR mutation rate and the higher number of previous lines of chemotherapy in the pembrolizumab monotherapy group might explain its poor ORR and PFS in the present study. In addition, although not statistically significant, PD-L1 expression was also lower in the pembrolizumab monotherapy group, which may also have contributed to poor response and PFS of the pembrolizumab monotherapy group. Moreover, only seven out of 18 patients died and 4 patients did not present with disease progression due to the short study period. Further follow-up and long-term studies could help confirm these findings.
In conclusion, given our randomized phase I/IIa clinical trials showing a promising ORR and PFS without severe AEs after SNK01 and pembrolizumab combination therapy compared with pembrolizumab alone in NSCLC patients, combination therapy with pembrolizumab and autologous NK cell therapy would potentially improve the therapeutic effects of pembrolizumab. This study also provides the basis for performing large-scale phase III clinical trials.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This prospective clinical trial was approved by the institutional review board (IRB, 2018-1479) of Asan Medical Center (Seoul, South Korea) and registered at the Clinical Research Information Service (CRIS, KCT0003463). Informed consent was obtained from all participants prior to enrollment. The trial was designed and conducted in accordance with the Helsinki Declaration and the Ethical Guidelines for Clinical Studies. Author Contributions Conceived and designed the analysis: Kim EJ, Rho JK, Choi CM. Collected the data: Kim EJ, Cho YH, Kim DH, Ko DH, Do EJ, Kim SY, Kim YM, Jung JS, Kang Y, Ji W, Lee JC, Rho JK, Choi CM. Contributed data or analysis tools: Kim EJ, Cho YH, Kim YM, Jung JS, Kang Y, Rho JK, Choi CM. Performed the analysis: Kim EJ, Cho YH, Kim DH, Ko DH, Do EJ, Kim SY, Ji W, Choi MG, Lee JC, Rho JK, Choi CM. Wrote the paper: Kim EJ, Cho YH, Kim YM, Choi MG, Rho JK, Choi CM. Provide NK cells: Kim YM, Jung JS, Kang Y. Fig. 1Clinical trial profile. In total, 20 patients were enrolled to the trial. Except for the first three and the last three patients (cohort 1), the remaining patients were randomly assigned to cohort 0 or 2. The pembrolizumab monotherapy group (cohort 0) received regular therapy with intravenous injection of pembrolizumab (200 mg) on the indicated time. The pembrolizumab plus SNK01 group (cohort 1 or 2) received pembrolizumab plus a total of 6 SNK01 infusions in 42 days, i.e., weekly infusion for 6 weeks. DLT, dose-limiting toxicity; MTD, maximum tolerated dose; NK, natural killer. 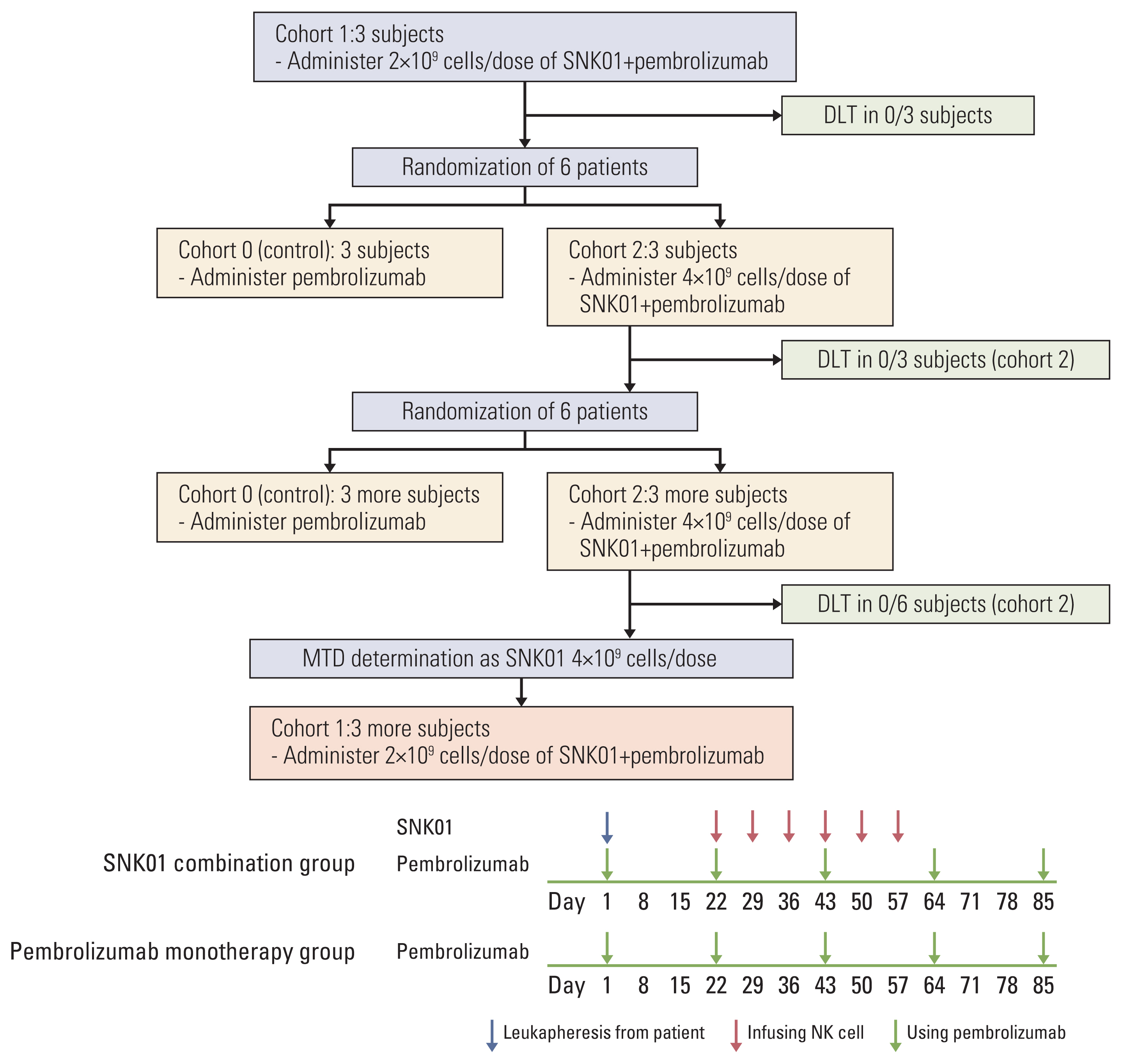
Fig. 2Characteristics of expanded NK cells. (A) The percentages of CD3−CD56+ NK cells, CD3+ T cells, CD20+ B cells, and CD14+ monocytes were analyzed flow cytometrically on freshly isolated positive cells using CliniMACS CD56 microbeads (D0; before expansion) and expanded NK cells for 17–18 days of culture (D17–18). (B) The fold expansion of the total cell population after 17–18 days of culture (D17–18). The expression levels of activating receptors, inhibitory receptors, chemokine receptors, perforin, and granzyme B were analyzed flow cytometrically among CD56+ gated cells after 17–18 days of culture. (C) The cytotoxic activity of expanded NK cells against the K562 and NCI-H2087 cell lines was measured via calcein-release assay at E:T ratios of 10:1 to 0.5:1 in triplicate. (D) The NK cell degranulation activity was measured flow cytometrically with % of CD56+CD107a+ in coincubation with K562 cells (E:T ratio=1:1), with phorbol 12-myristate 13-acetate/ionomycin treatment (as positive control, P.C.), or without treatment (negative control, N.C.). Dots represent the mean value of each patient from 5 to 6 cultures for clinical trial. Horizontal bars indicate the mean value, and each point represents mean±standard deviation. NK, natural killer. 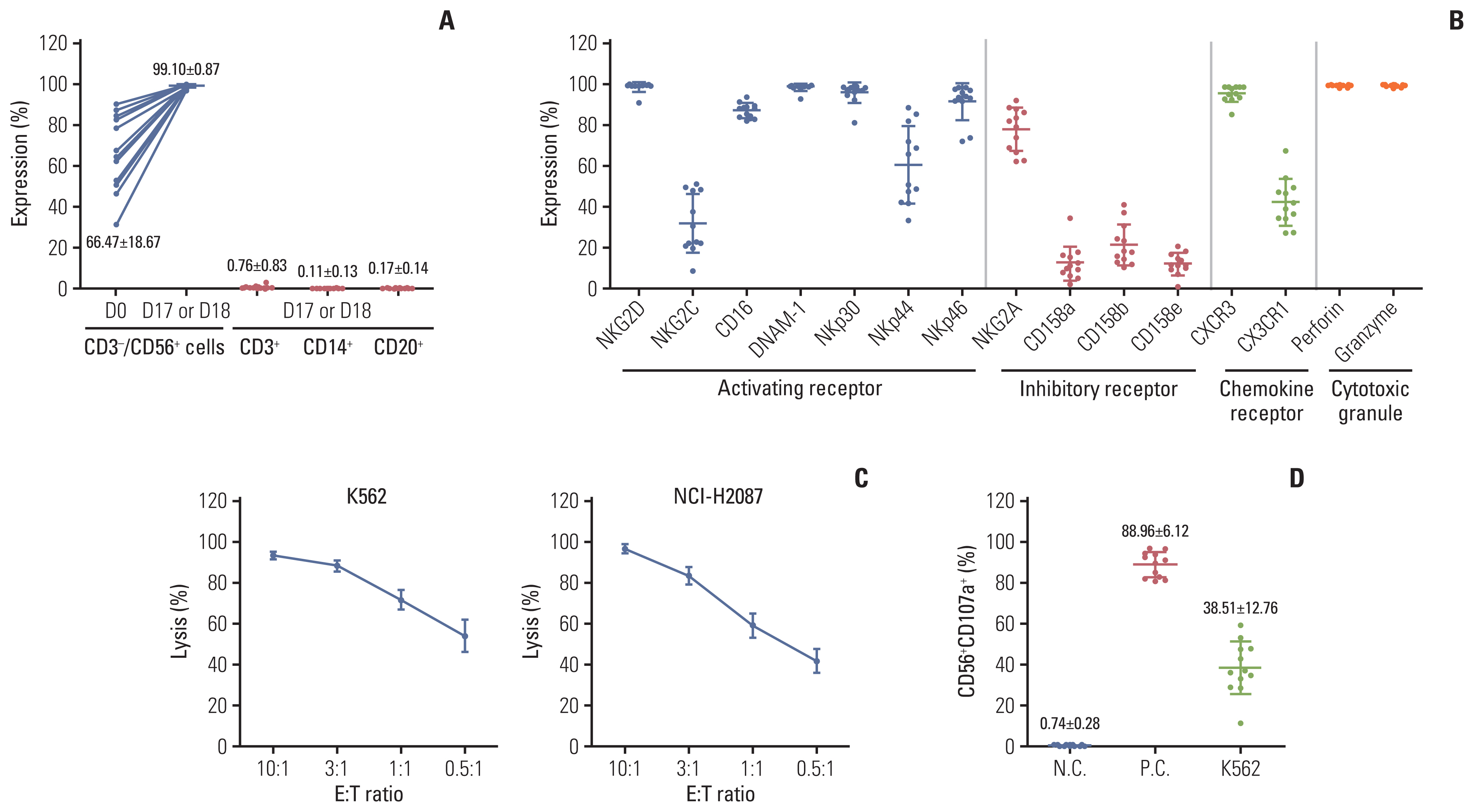
Fig. 3Progression-free survival analysis. Kaplan-Meier analysis was used to estimate the progression-free survival of each cohort (A) or group patients who received natural killer cell infusion (B). 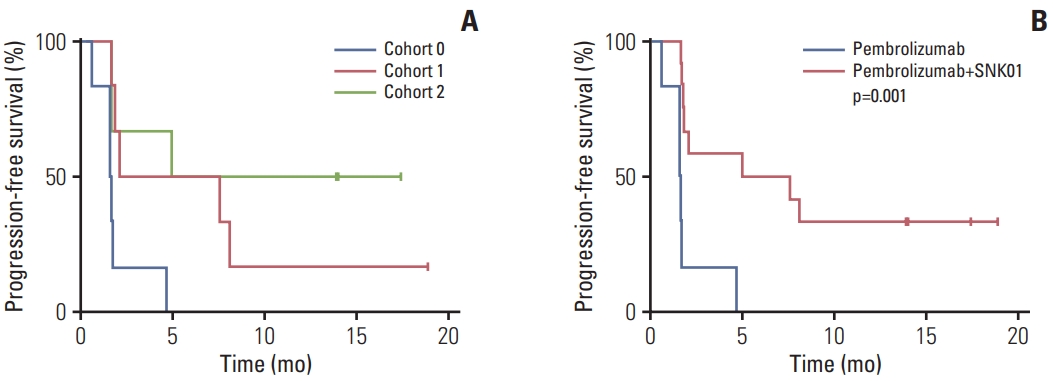
Table 1Baseline clinical characteristics of study patients (n=20) Table 2AEs reported in study patients (n=20) Table 3Comparison of efficacy outcomes between two treatment groups (n=18)
Values are presented as number (%) unless otherwise indicated. CI, confidence interval; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease. References1. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
2. Cosaert J, Quoix E. Platinum drugs in the treatment of non-small-cell lung cancer. Br J Cancer. 2002;87:825–33.
3. Tiseo M, Boni L, Ardizzoni A. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: does cisplatin versus carboplatin make a difference? J Clin Oncol. 2005;23:6276–7.
4. Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18.
5. Hui R, Garon EB, Goldman JW, Leighl NB, Hellmann MD, Patnaik A, et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: a phase 1 trial. Ann Oncol. 2017;28:874–81.
6. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
7. Rizzo A, Ricci AD, Brandi G. PD-L1, TMB, MSI, and other predictors of response to immune checkpoint inhibitors in biliary tract cancer. Cancers (Basel). 2021;13:558.
8. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019;4:e126908.
9. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–8.
10. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–46.
11. Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283:110–20.
13. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14:7.
14. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120.
15. Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12:eaaz5618.
16. Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–68.
17. Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130:2560–9.
18. Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1-negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 2019;9:1422–37.
19. Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, et al. PD/1-PD-Ls checkpoint: insight on the potential role of NK cells. Front Immunol. 2019;10:1242.
20. Lim SA, Kim TJ, Lee JE, Sonn CH, Kim K, Kim J, et al. Ex vivo expansion of highly cytotoxic human NK cells by cocultivation with irradiated tumor cells for adoptive immunotherapy. Cancer Res. 2013;73:2598–607.
21. Huang Z, Su W, Lu T, Wang Y, Dong Y, Qin Y, et al. First-line immune-checkpoint inhibitors in non-small cell lung cancer: current landscape and future progress. Front Pharmacol. 2020;11:578091.
22. Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. 2018;2018:6984948
23. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
24. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92.
25. Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124.
26. Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol. 2019;10:1205.
27. Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011;17:6287–97.
28. Shin MH, Kim J, Lim SA, Kim J, Kim SJ, Lee KM. NK cell-based immunotherapies in cancer. Immune Netw. 2020;20:e14.
29. Choi MG, Kim YJ, Lee JC, Rho JK, Choi CM. Efficacy of natural killer cell activity as a biomarker for predicting immunotherapy response in non-small cell lung cancer. Thorac Cancer. 2020;11:3337–45.
30. Tang SQ, Tang LL, Mao YP, Li WF, Chen L, Zhang Y, et al. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res Treat. 2021;53:339–54.
31. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306.
32. Naing A, Hajjar J, Gulley JL, Atkins MB, Ciliberto G, Meric-Bernstam F, et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer. 2020;8:e001754.
33. Wong HH, Halford S. Dose-limiting toxicity and maximum tolerated dose: still fit for purpose? Lancet Oncol. 2015;16:1287–8.
|
|