INTRODUCTION
The twenty-year prospective randomized trials have demonstrated that the treatment results of breast-conserving therapy (BCT) and modified radical mastectomy (MRM) were equivalent for early breast cancer (EBC, 1, 2). BCT can be perfected by using the free margins of the specimens, and an acceptable cosmetic result can be obtained following treatment with the appropriate radiation therapy (1).
Axillary lymph node dissection (ALND) continues to be the standard of care for BCS, but most of the morbidity related to BCS is associated with performing extensive surgical axillary dissection. In an effort to decrease the axillary morbidity, the technique of the sentinel node biopsy has been developed to provide surgeons with information that allows ALND to be avoided if the sentinel node is negative.
Radiotherapy after BCS reduces the local recurrence of breast cancer (1). Historically, whole breast tangential fields are used when for treating EBC, but they do not routinely identify the I-II levels of the axilla, given that this area has been surgically treated with ALND.
Currently, as the extent of axillary surgery decreases, the radiation dose and distribution within the axilla, as well as within the breast, becomes increasingly important. Several reports have shown that the traditional breast tangential fields fail to adequately treat the level I-II axillary lymph nodes (3, 4).
In the late 1980's, a real time, CT-linked, 3D treatment planning system called a CT simulator was developed and it allows full 3D viewing and planning of the patient. Because of the advantages for target identification and 3D dose calculation (5), the CT simulator is routinely used in clinical practice at several institutes for the treatment of various cancers. However, there are few reports concerning the therapeutic benefit of 3D CT-based simulation over 2D contour-based simulation in view of the local control and the overall survival for breast cancer.
We hypothesized that 3D CT-based simulation can more adequately define the breast parenchyma and the axillary volume at risk than can 2D contour-based simulation. An improved treatment outcome can be then obtained due to the appropriate axillary coverage, as well as to the homogenous dose distribution within the breast and axilla, with the help of 3D radiation treatment planning (RTP) after simulation. The purpose of this study is to evaluate the effect of the simulation method on the EBC recurrence with BCT.
MATERIALS AND METHODS
1) Patients
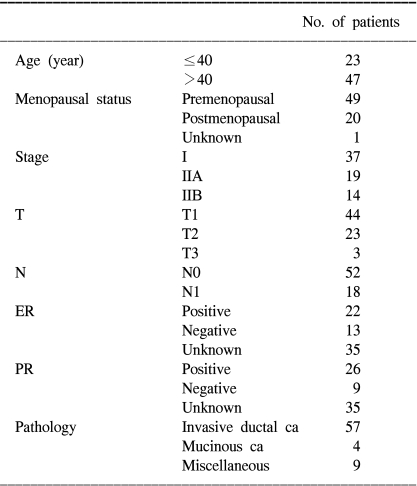
From September 1995 to February 2000, we identified 70 patients who suffered with pathologic stage I-II invasive breast cancer (IBC) by conducting a database review, and these 70 patients had undergone BCT at Kangnam St. Mary's Hospital. The pathologic stage was used to select the study population so as to avoid any interobserver variability in the assignment of the clinical stage. Tumor and lymph node staging were performed according to the 6th edition of the AJCC Cancer Staging Manual (6).
2) Surgical therapy
In all cases, excision of the primary tumor was macroscopically complete. If the surgical margins were positive, then reexcision was performed in most cases. Sixty-seven of the 70 patients who were treated during the study period underwent level I and II ALND, with or without sentinel lymph node biopsy, at the discretion of the surgeon. The median number of axillary nodes that was examined was 14 (range: 0 to 36). For the 3 patients without ALND, the lymph node staging was determined by a preoperative clinical examination such as performing breast sonogram. Because there was no evidence of axillary lymphadenopathy, they were classified as N0.
3) Systemic therapy
44 patients were treated with a cyclophosphamide-containing regimen. Adjuvant chemotherapy was offered to the patients showing histologic evidence of lymph node involvement and to some of the patients with lymph node-negative disease. Chemotherapy was given sequentially before radiotherapy to 43 patients and concurrently with radiotherapy to 1 patient. The oral 5-fluorouracil was given to 48 patients.
Tamoxifen treatment was considered for the patients with estrogen-receptor (ER) or progesterone-receptor (PR)-positive tumors after chemotherapy or for the patients who did not receive chemotherapy after surgery. Most of the patients to whom tamoxifen was offered were postmenopausal.
4) Radiation therapy
For the simulation, the patients were immobilized with an individualized vacuum mold (Chun-sung Co., Korea) with the ipsilateral arm abducted over 90°. Radiopaque wires were placed on the skin over the clinically selected superior, inferior, medial and lateral breast tangential field borders.
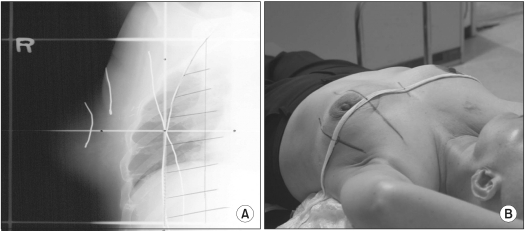
We conducted 2D contour-based simulation from September 1995 until August 1997. The treatment angles were determined by the radiopaque wires that coincided with the medial and lateral borders. A plaster mold contour was made using the axial center line of the body (Fig. 1).
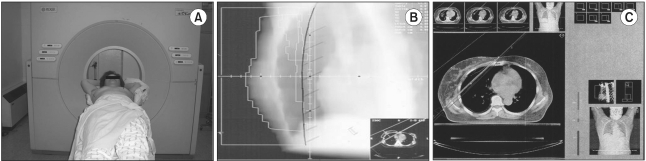
Between September 1997 and the end of the study period, 3D CT-based simulation was done using a AcQSim scanner (Picker international, Cleveland, OH, Fig. 2). Before determining the center of a radiation field, we first checked whether the breast tissues and level I-II axillary areas could be adequately covered. We delicately outlined the breast parenchyma and anatomic level l-ll axillary volume with including the surgical clips of the tumor bed on the axial CT images. Using the beam's eye view, an optimal tangential portal was determined that covered the target while it minimized the radiation to the normal tissue such as lung, heart and contralateral breast (Fig. 2).
In case of 2D simulation, we gave the same dose weight for each portal, so we could not expect the exact dose distribution. But for the 3D simulation, treatment-planning was carried out for an appropriate dose delivery and a homogenous dose distribution within the breast and axilla by using the RTP system (Helax, MDS, Nordian, Netherlands, Fig. 2). A dose volume histogram was used to measure the 95% prescribed dose coverage of the breast and the 90% prescribed dose coverage of the axillary areas.
Radiation was delivered on the breast using the tangential fields with 4 or 6-MeV photons via a linear accelerator (Siemens Inc., Concord, CA). The total dose was 48.6~50.4 Gy (median dose: 50.4 Gy), and this was delivered in 27~28 fractions over a 6 week period. A 0~9 Gy (median, 9 Gy) boost was delivered to the tumor bed with reduced fields with either photons or electrons.
5) Follow-up
All the patients were followed for at least the first 5 years after treatment. Any new carcinoma that was detected regionally or systemically was considered as a recurrence. The survival time was calculated from the date when the patient's disease was pathologically diagnosed, and disease-free survival was defined as the interval between the diagnosis of the primary tumor and the diagnosis of the recurrence or the last follow-up.
6) Statistical methods
The SPSS 12.0 software package (SPSS Inc., Chicago, IL) was used for the statistical analyses. Survival analyses were calculated by the Kaplan-Meier method, with comparisons among groups being performed with two-sided log-rank tests. All the tests were two-tailed, with p values of 0.05 or less being considered significant. The endpoints for analysis were the cumulative rates of recurrence and recurrence-free survival. Chi-square testing was used to compare the proportions of patients and the tumor characteristics, and the treatment variables between the 2D and 3D simulation groups.
RESULTS
2) Treatment outcome
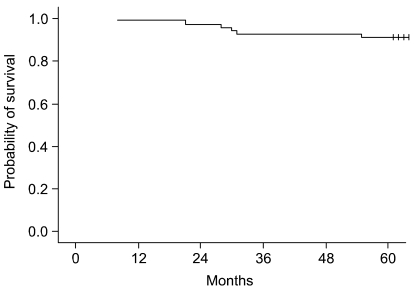
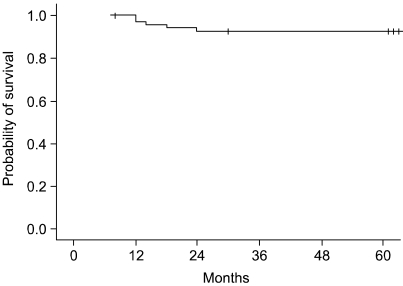
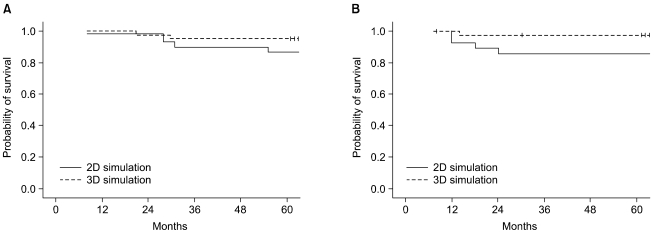
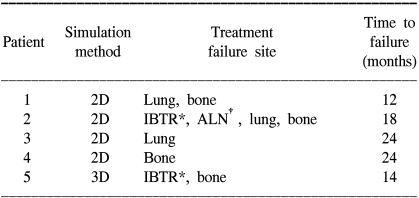
At a median follow-up time of 75 months (range: 5 to 110 months), the 5-year overall survival (OS) rate of all the treated patients was 91.4% (Fig. 3). For the 2D and 3D simulation groups, the 5-year OS rates were 89.2% and 95.1%, respectively (p=0.196, Fig. 5). Of the 70 patients who received BCT, 64 patients were alive at the last follow up. Four patients died of breast cancer and the remaining 2 patients died of unrelated causes, which were progression of colon cancer and aggravation of underlying liver cirrhosis. The five year DFS rate for all the patients was 92.7% (Fig. 4). For the 2D and 3D simulation groups, the 5-year DFS rates were 86.2% and 97.5%, respectively (p=0.0636, Fig. 5). Treatment failures were observed in 5 patients (4 in the 2D simulation group and 1 in the 3D simulation group) during follow up. Of the 4 patients who experienced treatment failures in the 2D simulation group, 3 patients showed only distant metastases as a cause of failure (1 in bone and lung at 12 months, 1 in bone at 24 months, and 1 in lung at 24 months) and the other patient showed both local and distant failure at 18 months. Only one patient in the 3D simulation group showed both local and distant failure at the 14 month follow-up. The patient characteristics of the 5 failed cases are shown in Table 4.
3) Factors affecting OS and DFS
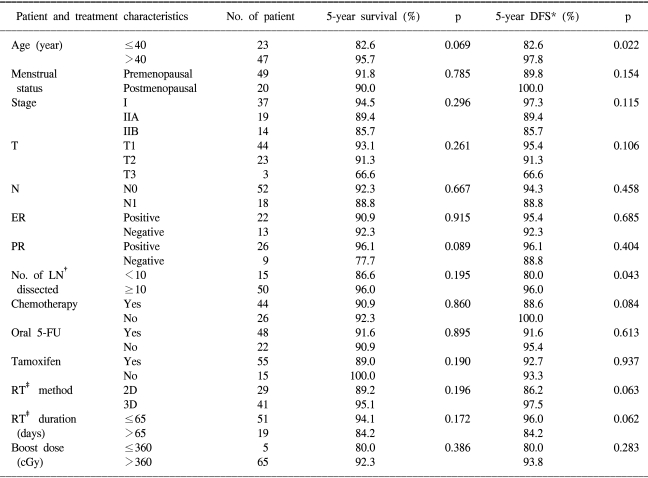
The prognostic factors for failure are shown in Table 5. There was no statistically significant prognostic factor for the 5-year OS rate. Yet the group aged over 40 years (p=0.069) and the patients who were positive for PR (p=0.089) showed a tendency for an increased survival duration.
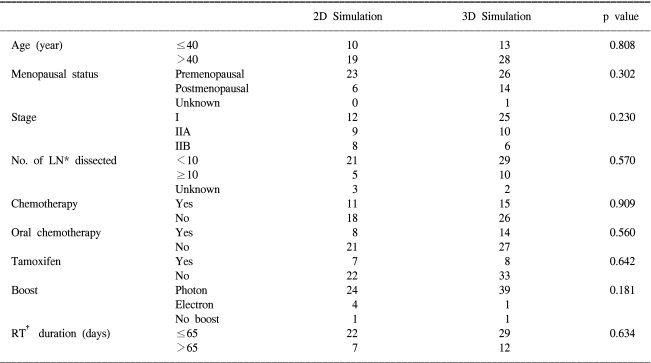
For the 5-year DFS rate, the group whose age was over 40 years (p=0.0226) and who more than 10 lymph node removed (p=0.0435) showed statistically significant results. Although it was marginal, a statistically significant difference was shown between the 2D simulation group and the 3D simulation group (p=0.0636). The RT duration was also a marginally significant prognostic factor in this study (p=0.0625).
DISCUSSION
BCT provides a satisfactory cosmetic result without compromising the local control or survival in comparison with MRM (1,2). A significant decrease of the recurrence rate has been demonstrated for patients treated with lumpectomy plus radiotherapy as compared with lumpectomy alone (1,18,22). As a consequence, BCS with postoperative radiation has replaced mastectomy for the majority of patients with EBC (7,8), and radiotherapy after BCS has played a significant role in BCT.
For decades, ALND has been the standard procedure in the surgical treatment of patient with breast cancer, and it was recommended both to treat potential nodal metastases and to help determine the adjuvant treatment (9). However, the use of ALND in women with breast cancer is associated with considerable morbidity. Karen et al (10). have analyzed 370 women with node negative breast cancer who underwent BCS with ALND and radiation therapy, and they reported arm symptoms in 80% of the patients. Schijven et al (11). have retrospectively compared the morbidity between ALND and sentinel node biopsy. They reported that an axillary procedure is the strongest and most consistent factor in explaining the differences in a variety of self-reported complaints and that axillary radiation therapy does not explain the lymphoedema by itself. Because of such problems, sentinel node biopsy has become the main procedure in BCS and some institutions have started to omit axillary dissection in those patients with clinically negative axillary lymph nodes (12~15). But there is a requirement for the surgeon to have a high level of skill for performing sentinel node biopsy so that the procedures should achieve a false negative rate of less than or equal to 5% (16,17). So, we cautiously suggest that postoperative radiation therapy of the axillary area should make up for any defect of the sentinel node biopsy.
The interest for having a well designed radiotherapy after BCS has recently increased with the current trend of the decreasing extent of axillary surgery. Advances in radiation therapy techniques are arriving via CT simulators and computer planning systems (19). With the improved anatomic delineation between the target volumes and the critical organ structures, the treatment fields can be designed to be more congruous to the areas that are at the highest risk (20,21). In spite of the various advantages of the CT simulator, there are few analyses of clinical data about the local control rate or survival for the patient undergoing 3D CT-based simulation.
Some reports have showed that standard tangential breast radiation fields failed to deliver an adequate therapeutic dose to the axillary level I-II lymph node anatomic volume (4,14). This problem can be solved with specific targeting of the anatomically defined axillary lymph node volumes, in addition to targeting the breast parenchymal volume, by means of 3D CT based-simulation and 3D RTP. For the case of 3D simulation, the boost field can include seroma that is formed near the tumor bed as well as at the surgical clips when directly viewing the axial image on the CT slices. The choice of the energy level for the electrons is determined by the thickness of the breast tissues. The depth of the lung from the skin surface should also be considered in this selection.
Our study showed that the patients with more than 10 dissected lymph nodes had a significantly improved DFS (p=0.0435). Costa et al (23). have reported that the ratio between the positive lymph nodes and the total dissected lymph nodes was the only independent predictor of relapse on multivariate analysis (p<0.001). Considering the importance of controlling axillary lymph node metastasis, we think achieving a decreased extent of ALND hands the task of controlling the axillary lymph node disease to well-designed radiotherapy. The age group over 40 years had superior DFS (p=0.0226), and similarly, Kim et al (24). supports this fact via the local-recurrence-free survival rate in his study on multivariate analysis (p=0.02).
In our data, among the patients with EBC who underwent BCT, the 3D CT-based simulation group had tendency for a reduced risk of recurrence. Considering that the pathway for spreading breast cancer is first the regional lymphatic area, appropriate axillary coverage by 3D CT-based simulation will make up for a sentinel node biopsy procedure. But even if our data showed a marginal significance for the DFS between the two groups, it may be not due to the difference of the loco-regional recurrence rate that resulted from the simulation method, but rather, it was the result of the systemic metastasis. We cannot definitely conclude whether 3D CT-based simulation might improve the DFS and reduce the risk of recurrence as compared with 2D contour-based simulation due to the relatively small group of patients and the short follow-up period. To evaluate the effect of the simulation method, another study is needed with a reliability estimated larger group and a longer follow-up period.
CONCLUSIONS
As the extent of axillary surgery decreases, the radiation dose and distribution within the breast and axilla becomes increasingly important for current therapy planning and the future analysis of results. 3D CT-based simulation appears to be a useful procedure for the individualized optimized tangential irradiation during BCT. To confirm our results, we need further studies with more patients and further long term follow-up. Although the number of patients in our study was too small to draw firm conclusions concerning the effects of the simulation method on recurrence after radiotherapy for the early breast cancer, we expect that the improved results of patients who undergo 3D CT-based simulation will be shown in the near future.