AbstractPurposeMetachronous brain-only oligorecurrence in patients with non–small cell lung cancer (NSCLC) is a rare event with favorable prognosis, but the clinical outcome has not been fully determined. We retrospectively analyzed clinical outcomes and prognostic factors in metachronous brain-only oligorecurrence in patients with NSCLC who underwent definitive treatment.
Materials and MethodsWe reviewed 4,437 NSCLC patients without oncogenic driver mutations who underwent definitive treatment between 2008 and 2018. Among them, we identified 327 patients who developed 1 to 5 brain metastases with or without systemic metastasis. Of the 327 patients, 71 had metachronous brain-only oligorecurrence without extracranial progression and were treated with local therapy to the brain. Overall survival (OS), progression-free survival (PFS), and prognostic factors affecting OS were analyzed.
ResultsThe median OS was 38.9 months (95% confidence interval [CI], 21.8 to 56.1 months) in 71 patients. The 2-year OS rate was 67.8% and the 5-year OS rate was 33.1%. The median PFS was 25.5 months (95% CI, 12.2 to 14.4 months). The longest surviving patient had a survival period of 115 months. Through multivariate analysis, Eastern Cooperative Oncology Group ≥ 1 (hazard ratio, 5.33; p=0.005) was associated with poor survival. There was no significant difference in OS between patients with local therapy and those with local plus systemic therapy (18.5 months vs. 34.7 months, p=0.815).
IntroductionIn National Comprehensive Cancer Network guidelines, recommendations for treatment of early-stage non–small cell lung cancer (NSCLC) involve definitive treatment, including surgery and concurrent chemoradiation (CRT) due to their greatest opportunity for a cure [1]. Brain metastases are a common complication in NSCLC patients. However, there are few data on incidence of brain metastasis in early-stage NSCLC. One retrospective study reported that the incidence of brain metastasis in postoperative early-stage NSCLC was low, approximately 6% [2]. After CRT for stage III NSCLC patients, 13%–15% of patients develop symptomatic brain metastases within the first year [3]. Approximately 10% of patients at the time of diagnosis [4] and approximately 10%–30% of patients during the disease course experience brain metastases [5]. Brain metastases can cause neurologic symptoms, including headaches, focal neurologic deficits, cognitive dysfunction, and seizures, resulting in a negative effect on the quality of life and, ultimately, poor survival. In addition, most patients with brain metastases have synchronous extracranial metastases [6]. Therefore, the median overall survival (OS) for recurrent or metastatic stage IV patients was less than 1 year, with a median progression-free survival (PFS) of approximately 5 months [7].
Hellman and Weichselbaum [8] first proposed the concept of oligometastses as 1–5 metastatic or recurrent lesions with active primary lesions. Oligometastses typically are treated with metastasis-directed local treatment, such as surgery or stereotactic radiosurgery (SRS), which can result in long-term survival or even cure [8]. In contrast, Niibe and Hayakawa [9] proposed the concept of oligorecurrence as 1–5 metastatic or recurrent lesions with controlled primary lesions. Similarly, oligorecurrence should be treated with metastasis-directed local treatment and is considered to have a better prognosis than oligometastases [9]. It has been reported that synchronous oligometastases are associated with worse clinical outcomes compared with metachronous oligometastases. Therefore, it is important to distinguish between oligometastases and oligorecurrence. Recently, the European Society for Radiotherapy and Oncology (ESTRO) and European Organization for Research and Treatment of Cancer (EORTC) OligoCare Project developed a comprehensive system for characterization and classification of oligometastatic disease [10].
Brain metastases in NSCLC are managed with well-established approaches including local treatment, cytotoxic systemic chemotherapy, molecular targeted therapies, and immune checkpoint inhibitors. However, metachronous brain-only oligorecurrence NSCLC patients without oncogenic driver mutations who underwent definitive treatment are rare, and there is limited data on appropriate treatment strategies and long-term clinical outcomes. In this study, we investigated the clinical outcomes of metachronous brain-only oligorecurrence in patients with NSCLC without oncogenic driver mutations who previously underwent definitive treatment with long-term follow-up.
Materials and Methods1. PatientsThe DNA extracted from formalin-fixed paraffin-embedd We retrospectively reviewed 4,437 patients with epidermal growth factor receptor (EGFR) mutation wild type and anaplastic lymphoma kinase (ALK) rearrangement-negative NSCLC stage I–IIIC (according to the American Joint Committee on Cancer, 8th edition, TNM staging system) who underwent definitive treatment such as surgery, CRT, or definitive RT at Samsung Medical Center, Korea, between April 2008 and April 2018. Regardless of systemic metastases, 327 patients showed one to five brain metastases on brain magnetic resonance imaging (MRI) and were treated with surgery, whole-brain radiotherapy (WBRT), or SRS. Of the 327 patients, 71 had metachronous brain-only oligorecurrence without extracranial progression and were treated with local therapy, while 17 had synchronous oligorecurrence (maximum 6-month interval between diagnosis of brain oligorecurrence and diagnosis of primary lung cancer) (Fig. 1). Oligorecurrence was confirmed by radiologic imaging such as computed tomography (CT) of the chest, abdomen, and pelvis; MRI of the brain; or positron emission tomography–CT.
2. End point definition and response evaluationOS was calculated from the date of treatment for brain metastases to the date of death from any cause. PFS was calculated from the date of treatment for brain metastases to the date of extracranial metastases or death due to any cause. Disease-free interval (DFI) was calculated from the date of definitive treatment for primary lesions to the date of diagnosis for brain-only oligorecurrence. The date of definitive treatment for primary lesions was defined as the date of surgery or CRT.
3. Statistical analysesThe cut-off date for data collection was September 14, 2020. Descriptive statistics were used to summarize patient and tumor characteristics and treatment history. Clinical outcomes of OS, PFS, and DFI were evaluated based on the Response Evaluation Criteria in Solid Tumors ver. 1.1 by the investigators. DFI, PFS, and OS were assessed by the Kaplan-Meier method and p-values from a log-rank test. Univariate analysis of prognostic factors was performed by the log-rank test for OS, and multivariate analysis was performed using Cox proportional hazards models. Multivariate analysis was used in factors that were significant (p < 0.05) or exhibited a non-significant trend toward significance (p < 0.25) on univariate analysis. IBM SPSS Statistics ver. 25 (Armonk, NY) was used for statistical analysis.
Results1. Patient characteristicsSeventy-one patients (71/4,437, 1.6%) who met the criteria for one to five metachronous brain-only oligorecurrences were identified. The median age was 64.8 years (range of 40 to 81 years). The Eastern Cooperative Oncology Group (ECOG) performance score (PS) of most patients was 0 or 1. Adenocarcinoma (83%) was the main histological subtype. The numbers of initial stage I, II, and III NSCLC patients were 11 (15%), 17 (24%), and 43 (61%), respectively. Sixty-three patients (89%) with stage I, II, IIIA, or IIIB were treated by surgery, and seven patients (10%) with stage IIIB or IIIC were treated with CRT with curative intent. For brain metastases, 58 (81.7%) patients were treated with local treatment (SRS, surgery), five (7.0%) were treated with WBRT, eight (11.3%) with local treatment (SRS, surgery) and WBRT. Forty-four patients (62%) had a longer than 12-month interval from definitive treatment to initial brain recurrence, and 46 patients (65%) had single brain metastasis. Only 10 patients (14%) received local treatment to the brain along with systemic chemotherapy (Table 1).
2. OS and prognostic factor analysisThe median follow-up was 41.2 months (19.2 to 145.4). The median OS was 38.9 months (95% confidence interval [CI], 21.8 to 56.1) in 71 patients. The 2-year OS rate was 67.8% and the 5-year OS rate was 33.1% (Fig. 2). The median OS of initial stages I, II, and III were 59.7 months, 24.0 months, 37.3 months, respectively (p=0.195). Univariate analysis revealed ECOG PS (p=0.005), histological subtype (p=0.040), and treatment method for metastatic brain lesion (p=0.081) as significant factors associated with OS (Table 2). Multivariate analysis showed ECOG PS (p=0.005) as significant independent prognostic factors for poor OS (Table 3). However, there were no clear associations between OS and initial stage (p=0.685), definitive treatment method for the primary site (p=0.342), interval from initial treatment to brain metastasis (p=0.095), and number of metastatic brain lesions (p=0.226). Of note, seven patients (10%) survived more than 5 years from the date of treatment for brain metastases. The longest surviving patient had a survival period of 115 months.
3. Progression-free survivalThe median PFS was 25.5 months (95% CI, 16.6 to 34.4). The 2-year PFS rate was 50.2% and the 5-year PFS was 17.4% (Fig. 3). Twenty patients developed extracranial metastases after local treatment for metastatic brain lesions. Extracranial metastatic sites were lung to lung metastasis in six patients, regional lymph nodes in eight patients, bone in seven patients, adrenal gland in one patient, liver in one patient, kidney in one patient, and mesentery mass in one patient. The median disease-free survival for brain metastasis was 13.3 months (95% CI, 12.2 to 14.4). The 1-year DFI rate was 62.0% and the 2-year DFI rate was 19.7% (Fig. 4). The median DFI values of initial stages I, II, and III were 25.7 months, 12.9 months, 13.3 months, respectively (p=0.091).
4. Local treatment with or without systemic chemotherapy for brain metastasesOf the 71 patients, 61 received local treatment alone at the time of diagnosis for brain-only oligorecurrence and metastatic brain tumors were well controlled by local treatment without progression or leptomeningeal seeding. Among them, 15 patients developed extracranial metastases during follow-up and were treated with systemic chemotherapy. Only 10 patients received local treatment followed by systemic chemotherapy at the time of diagnosis for brain-only oligorecurrence; nine patients received a platinum-based combination chemotherapy, six of whom received pemetrexed and cisplatin for 2 to 6 cycles. The median OS of the patients receiving local treatment along with systemic chemotherapy at the date of treatment for brain metastases (n=10) was shorter than that of those treated with local treatment alone (n=46). However, there was no significant difference in OS according to systemic chemotherapy (p=0.815).
5. Comparison between synchronous and metachronous brain-only oligorecurrenceWe identified 17 patients with synchronous oligorecurrence, which was defined as a maximum 6-month interval between diagnosis of brain oligorecurrence and diagnosis of primary lung cancer. The median OS was 38.9 months (95% CI, 21.8 to 56.1) in 71 patients with metachronous brain-only oligorecurrence, whereas the median OS was 52.0 months in 17 patients with synchronous brain-only oligorecurrence (p=0.575). There was a numerically longer OS in patients with synchronous brain-only oligorecurrence.
DiscussionThe current study demonstrated a median OS of 38.9 months (95% CI, 21.8 to 56.1) in 71 patients who underwent definitive treatment for primary lesions and received local treatment for metachronous brain-only oligorecurrence. The median PFS was 25.5 months (95% CI, 12.2 to 14.4). The 2-year OS rate was 67.8%, approximately 33% of the patients survived longer than 5 years, and 17% of the patients had no extracranial relapse for more than 5 years, suggesting that patients with brain-only oligorecurrence after curative intent treatment experience long-term survival with local treatment.
Previous studies reported favorable clinical outcomes of local treatment for oligometastases in NSCLC patients [11–13]. However, until recently, the standard distinction between oligometastases and oligorecurrence had not been well-established. Furthermore, there was no definitive distinction between synchronous and metachronous brain-only oligorecurrence NSCLC due to its rarity. Therefore, most studies included patients with synchronous oligometastases with uncontrolled primary lesions. In 2020, the ESTRO and EORTC OligoCare Project developed a comprehensive system for characterization and classification of oligometastatic diseases [10]. One Japanese group previously reported a median OS of 41 months in brain-only oligorecurrence NSCLC patients treated with local radiation therapy [14], which is consistent with our results. However, they did not distinguish between synchronous and metachronous oligorecurrence, and the number of patients was small. In this study, we separately analyzed patients with metachronous and synchronous oligorecurrence. Although there was no significant difference in OS between the two cohorts, it was important to distinguish these two recurrence patterns to explore treatment strategies and prognostic factors.
This study is unique because we excluded patients with oncogenic drivers, in whom many targeted agents penetrating the blood brain barrier are available, especially for EGFR- and ALK-positive NSCLC, which can overestimate the clinical outcome. Even without oncogenic driver mutations, patients with brain-only oligorecurrence exhibit long-term survival with local therapy. We also found that the incidence of brain-only oligorecurrence in this cohort was 1.6% (71/4,437), with a median DFI of 13.3 months. Most patients with stage II or III developed brain metastases around 12 months after definitive treatment. Considering that brain metastases were concurrent with extracranial progression, the incidence increased, indicating that proactive surveillance of brain metastasis should be considered. We also found that ECOG ≥ 1 (hazard ratio, 5.33; 95% CI, 1.66 to 17.13; p=0.005) was poor prognostic factors in metachronous brain-only oligorecurrence patients. In contrast, factors of initial stage, histological subtype, definitive treatment type for primary sites, interval time to brain metastasis, number of metastatic brain lesions, treatment method for brain metastasis, and systemic chemotherapy were not correlated. The underlying biology for metachronous brain metastases has yet to be determined. Given the limited patient numbers, further investigations are necessary to identify traits of patients who develop brain-only oligometastases. Along with local therapy to the brain, 14% of patients (10/71) received systemic chemotherapy to prevent further systemic relapse. Although we did not determine any significant difference in clinical outcomes between patients receiving local therapy alone and those treated with local therapy and systemic chemotherapy (18.5 months vs. 34.7 months, p=0.815), the role of systemic chemotherapy in brain-only oligorecurrence remains controversial. Based on our results, local therapy alone may be a reasonable approach for management of this patient population.
Our study has limitations. This is single-center retrospective study, leading to bias. In addition, given the location of a referral center, more aggressive treatment with active surveillance might affect the clinical outcomes compared with those in a community hospital. Nevertheless, to the best of our knowledge, this study is the largest with long-term follow-up analyzing the rare event of metachronous brain-only oligorecurrence in NSCLC patients.
Metachronous brain-only oligorecurrence NSCLC patients who underwent definitive treatment experienced long-term survival with local therapy, highlighting the rare but unique patient population. The role of systemic chemotherapy in this patient population requires further investigation.
NotesEthical Statement This study was approved by Institutional Review Board (IRB No. 2020-12-071) at Samsung Medical Center and individual consent for this retrospective analysis was waived. Author Contributions Conceived and designed the analysis: Kim H, Ahn MJ. Collected the data: Kim H, Park S, Jung HA, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Contributed data or analysis tools: Kim H, Park S, Jung HA, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. Performed the analysis: Kim H, Ahn MJ. Wrote the paper: Kim H, Ahn MJ. Fig. 1Flow diagram of the study population. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer; WT, wild-type. 
Fig. 2Kaplan-Meier curves of overall survival (OS) for the local treatment with or without chemotherapy in metachronous brain-only oligorecurrence in patients. CI, confidence interval. 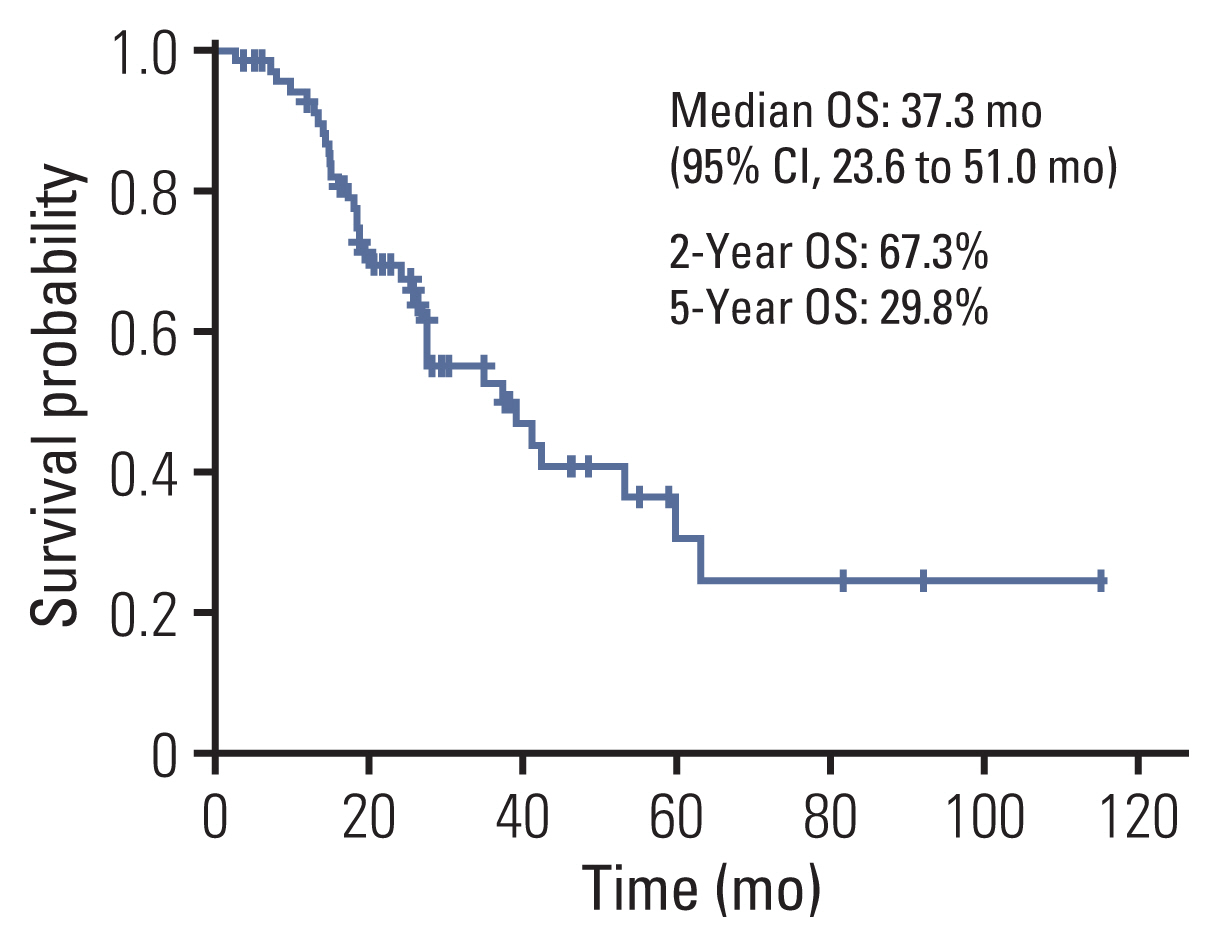
Fig. 3Kaplan-Meier curves of progression-free survival (PFS) for local treatment with or without chemotherapy in metachronous brain-only oligorecurrence in patients. CI, confidence interval. 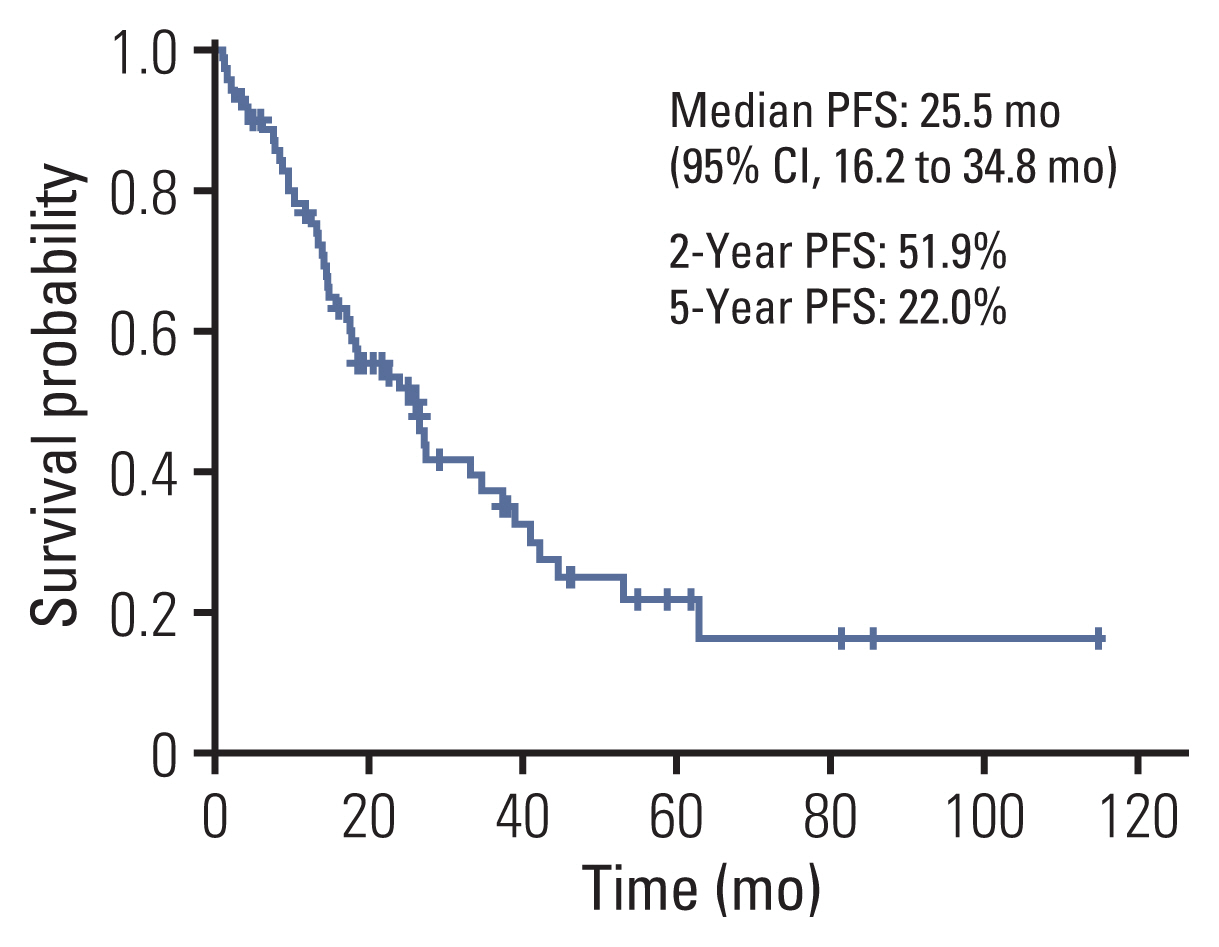
Fig. 4Kaplan-Meier curves of disease-free interval (DFI) for local treatment with or without chemotherapy in metachronous brain-only oligorecurrence in patients. CI, confidence interval. 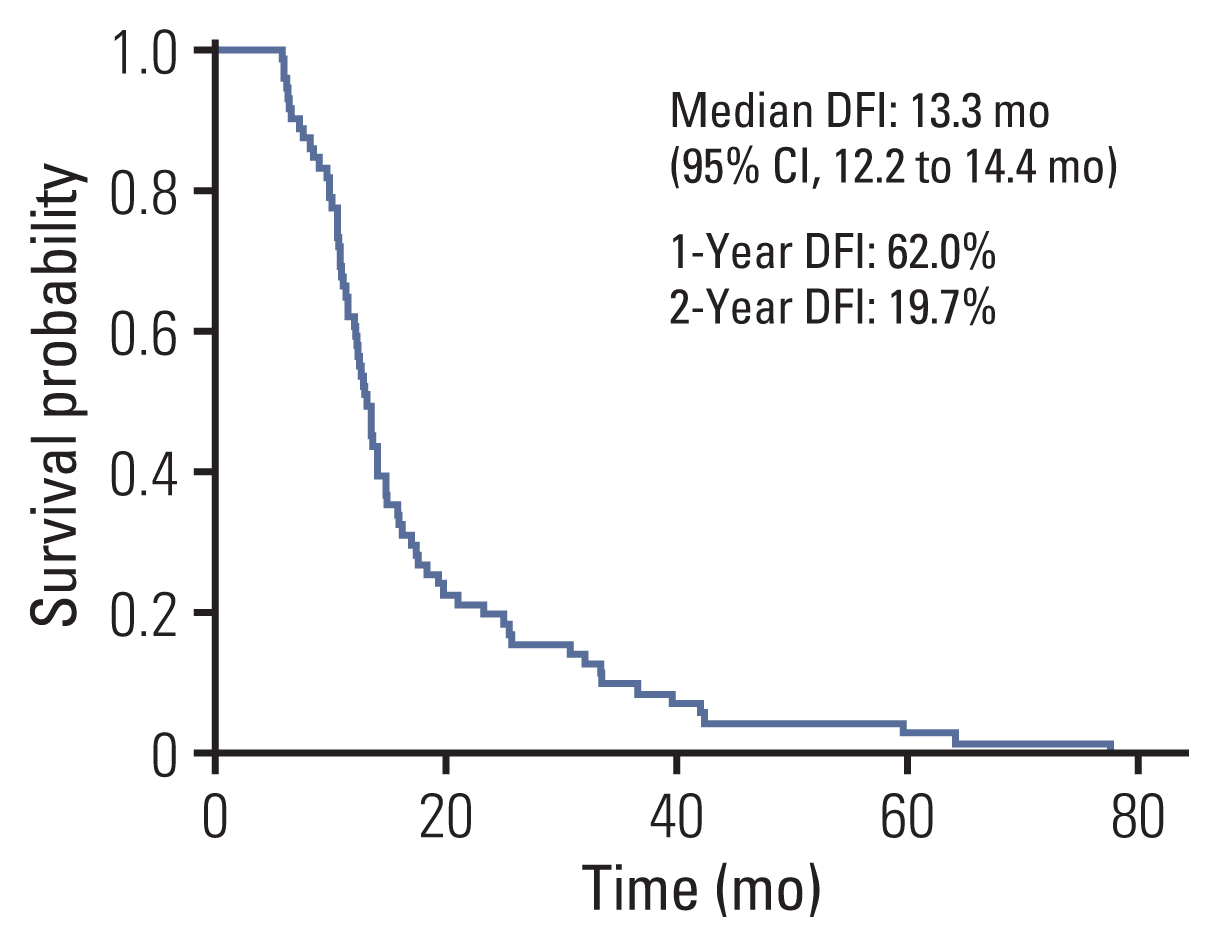
Table 1Patient characteristics Table 2Univariate analysis of overall survival Table 3Multivariate analysis of overall survival References1. National Comprehensive Cancer NetworkNon-small cell lung cancer (version 8.2020) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [cited 2020 Sep 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
2. O’Dowd EL, Kumaran M, Anwar S, Palomo B, Baldwin DR. Brain metastases following radical surgical treatment of non-small cell lung cancer: is preoperative brain imaging important? Lung Cancer. 2014;86:185–9.
3. Gaspar LE, Chansky K, Albain KS, Vallieres E, Rusch V, Crowley JJ, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005;23:2955–61.
4. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–705.
5. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14.
7. Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91.
9. Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107–11.
10. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–28.
11. de Vin T, Engels B, Gevaert T, Storme G, De Ridder M. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25:467–71.
12. Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2014;15:346–55.
13. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–65.
14. Niibe Y, Nishimura T, Inoue T, Karasawa K, Shioyama Y, Jingu K, et al. Oligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: a multi-institutional study of 61 subjects. BMC Cancer. 2016;16:659.
|
|
||||||||||||||||||||||||||||||||||||||||