AbstractPurposeOccupational exposure to pesticides is thought to be associated with lung cancer, but studies have yielded conflicting results. We performed a propensity score (PS) based analyses to evaluate the relationship between occupational exposure to pesticides and lung cancer risk in the Korea National Cancer Center community-based cohort study (KNCCCS).
Materials and MethodsDuring the follow-up period, 123 incidental lung cancer cases were identified, of the 7,471 subjects in the final statistical analysis. Information about occupational exposure to pesticides and other factors was collected at enrollment (2003–2010). Cox proportional hazards regression analyses were conducted. Four PS-based approaches (i.e., matching, stratification, inverse probability-of-treatment weighting, and the use of the PS as a covariate) were adopted, and the results were compared. PS was obtained from the logistic regression model. Absolute standardized differences according to occupational exposure to pesticides were provided to evaluate the balance in baseline characteristics.
ResultsIn the Cox proportional hazards regression model, the hazard ratio (HR) for lung cancer according to occupational exposure to pesticides was 1.82 (95% confidence interval [CI], 1.11 to 2.98). With all the propensity score matching (PSM) methods, the HRs for lung cancer based on exposure to pesticides ranged from 1.65 (95% CI, 1.04 to 2.64) (continuous term with PSM) to 2.84 (95% CI, 1.81 to 4.46) (stratification by 5 strata of the PS). The results varied slightly based on the method used, but the direction and statistical significance remained the same.
IntroductionPesticides, including herbicides, insecticides, fungicides, fumigants, and rodenticides, are important chemical agents widely used in the agricultural sector. Pesticides can, however, also be toxic to humans, and it has been estimated that approximately 250,000 people worldwide die of pesticide poisoning each year [1]. Long-term occupational exposure to pesticides has been associated with an increased risk of lung cancer. Lung cancer is the leading cause of cancer-related death worldwide, including in South Korea. According to the annual 2017 report on cause of death statistics from the Korea National Statistics Office (KNSO), lung cancer mortality increased from 28.7 per 100,000 people in 2006 to 35.1 per 100,000 people in 2016 [2].
Although smoking is a known major risk factor for lung cancer that has been identified in many studies, exposure to pesticides also contributes to the occurrence of lung cancer [3]. Increased lung cancer-related mortality among pesticide applicators has been reported [4–8], suggesting the possibility that exposure to pesticides may increase the risk of lung cancer among farmers [9]. In addition, previous cohort studies reported positive associations between exposure to pesticides and the occurrence of lung cancer [9–13].
Exposure to pesticides has caused lung tumors in rodent bioassays, but the epidemiologic data supporting an association between pesticides and lung cancer risk in humans are mixed [10,14]. Jones et al. [12] reported an increased lung cancer incidence among male pesticide applicators with high pesticide exposure in lifetime days (diazinon: rate ratio, 1.60; 95% confidence interval [CI], 1.11 to 2.31; p-trend=0.02). However, Silver et al. [13] found no association of the incidence of lung cancer with either lifetime days or intensity-weighted lifetime days of pesticide use. In addition, a follow-up study that was part of the Agricultural Health Study (AHS) provided additional evidence supporting a positive association between pesticide use and lung cancer risk [9].
Although there have been reports on the risk of lung cancer due to pesticide exposure in several studies, previous studies have used limited data with inadequate control of covariate adjustments. To reduce confounding by numerous patient characteristics, propensity score (PS) methods are increasingly being used in many studies as an alternative to conventional covariate adjustment to investigate the effects of risk factors and include stratification, matching, inverse probability weighting (IPW), and the use of the PS as a covariate in a conventional regression model [15,16]. Therefore, we examined the association between occupational exposure to pesticides and lung cancer incidence in a community-based cohort study and compared the results of various PS methods and conventional models that adjust for covariates.
Materials and Methods1. Data source and study populationThis study was performed using data from the Korea National Cancer Center community-based cohort study (KNCCCS), a community-based cohort study conducted from 1993 to 2010 in Korea. The methods of the study have been described in detail in the published study protocol [17]. All participants (16,304 men and women) were over 20 years old, with an average age at cohort entry as follows: 58.6±12.4 years for men (n=6,302) and 57.7±13.3 for women (n=10,002). Data were collected through a face-to-face interview by well-trained interviewers using a questionnaire, and health outcomes were also investigated. The questionnaires were administered by trained interviewers and focused on demographic characteristics, past medical history, family history of cancer, history of medication use, dietary habits, smoking and alcohol consumption habits, physical activity, occupational history, history of exposure to acupuncture and blood transfusion, history of exposure to pesticides and electromagnetic fields and reproductive history for women. Trained staff, including interviewers, were provided appropriate instructions and guidelines about recruiting participants, and they asked the participants for consent prior to participation in the study. All study participants were followed until 2017 through linkage with the Korean Central Cancer Registry for cancer incidence. All participants in this cohort were linked to mortality data from Statistics Korea up to 2017.
Fig. 1 shows the flow diagram of the selection of the study population. Of the 16,304 subjects in the KNCCCS, 9,448 men and women were eligible for measurement of pesticide exposure. After exclusion of 2,095 subjects who had a history of cancer before an entry in the KNCCCS (n=244), missing data on pesticide-related variables such as frequency of pesticide use and duration (n=1,311), smoking status (n=194), and other covariates such as alcohol consumption status, education, obesity, physical activity and job (n=346), we included 7,471 subjects in the final statistical analysis. For propensity score matching (PSM) analysis, 4,112 subjects were ultimately included after excluding 3,359 unmatched subjects.
2. History of exposure to pesticidesInformation on exposure to pesticides was collected between 2003 and 2010 by trained interviewers. Participants were asked to report all pesticides used in the year prior to the interview and the frequency and duration of pesticide use. The history of exposure to pesticides was assessed using the following four questions: ‘In your lifetime, have you ever used pesticides?’, ‘Have you worked in agriculture in the past?’, ‘Are you currently working in agriculture?’, and ‘Have you worked in agriculture?’. Occupational exposure to pesticides was defined as having experience using pesticides and working in agriculture in the present.
3. Definition of lung cancerThe cohort has been followed up annually to identify new cancer cases from the date of the baseline survey. It has been an exclusively register-based passive follow-up, and the participants have not been recontacted. The principal outcome variable was incident lung cancer, based on data from the National Cancer Registry. The Korean Ministry of Health and Welfare started a nationwide, hospital-based cancer registry in 1980 [18]. More than 180 hospitals are currently participating, and the data cover approximately 99% of new cancer cases in Korea. Cases were identified through a personal identification number and other usual identification variables, such as names and addresses. Cancer cases were classified according to the International Classification of Diseases, 10th edition (ICD-10), and lung cancer was coded as C33–34 [18,19].
4. Statistical analysesWe examined differences of baseline characteristics such as pesticide use, current job status, pesticide-related variables such as the frequency and duration of pesticide use, smoking status, age, study areas, year of entry into the cohort, education achievement, alcohol consumption status, physical activity, and obesity.
Follow-up started at enrollment and continued until a lung cancer diagnosis or censoring. Censoring occurred at the date of death or a diagnosis of any other cancers or the end of follow-up (December 31, 2017).
We conducted crude and multiple Cox proportional hazards regression, including the following covariates that are well-known risk factors for lung cancer: age, sex, smoking status (nonsmoker, < 30 pack-years, ≥ 30 pack-years), alcohol consumption (0 g/day, < 24 g/day, ≥ 24 g/day), obesity (body mass index [BMI]; < 25 kg/m2, ≥ 25 kg/m2), physical activity (< 5 days/wk, ≥ 5 days/wk), education level (illiterate, middle school or less, high school, and college or more), and study area (Sancheong, Changwon, Chuncheon, Chungju, and Haman).
For PS-based analyses, we calculated the PS. The PS was the probability that an individual would have had a history of occupational exposure to pesticides based on personal demographic and lifestyle information and was obtained from the logistic regression model adjusted for these variables: age, sex, smoking status, alcohol consumption, obesity, physical activity, education level, and study area as follows; First, we applied a 1:1 PSM technique, which is an eighth-digit-to-first-digit greedy matching method, as elucidated previously. Unmatched cases are eliminated to ensure the best matching. To evaluate the balance in baseline characteristics between those who had ever and never used pesticides after PSM, we determined the absolute values of the standardized differences. Differences less than 0.1 were considered negligible. Second, we constructed weighted logistic regression models that showed the adjusted effect of exposure to pesticides after stratification into five strata according to the PS quintiles. Additionally, a logistic regression model using the PS as a covariate (a continuous variable and a categorical variable by quintiles), which is similar to traditional regression analyses, was constructed. Finally, two major PS-weighting methods, which are standardization methods depending on the establishment of a standard population, were adopted. One such method involved inverse probability-of-treatment weighting (IPTW). This model considers the total study subjects as the standard population and uses weights of (1/PS) for those who have used pesticides and [1/(1–PS)] for those who have not used pesticides.
Finally, we applied the Cox proportional hazards regression to determine the effect of occupational exposure to pesticides on lung cancer risk, using the following models: crude and adjusted models, PSM, regression adjusted with the PS, and PS weighting. Standardized differences were calculated using the macro ‘tableone’ written in R statistical software and developed by Yoshida et al. [20].
Additionally, time-lag analyses after excluding subjects with a follow-up period of equal or less than 3 years were performed.
All statistical analyses were performed with SAS software ver. 9.3 (SAS Institute Inc., Cary, NC) and R ver. 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria) and were two-sided with a significance level of p < 0.05.
ResultsThe median follow-up period for subjects (n=7,471) eligible for the Cox proportional hazards regression analysis was 10.43 years (interquartile range [IQR], 8.5 to 12.48). A total of 123 primary lung cancer cases had been diagnosed since KNCCCS enrollment, and the median number of years of follow-up was 5.9 (IQR, 2.73 to 9.05).
The baseline characteristics of the study subjects are presented in Table 1. The mean age of the study subjects was approximately 60 years. The study population had higher proportions of, pesticides user, farm workers, subjects with BMI less than 25 kg/m2, and with a low education levels. Most of women were never-smokers (92.53%) and never-drinkers (76.05%). In contrast, most of men were former- or current smokers (79.4%) and drinkers (75.76%) (Table 1).
Table 2 shows the associations between occupational pesticide exposure-related variables and lung cancer risk. After stratification by sex, the association between occupational exposure to pesticides and lung cancer risk was statistically significant for men (HR, 2.40; 95% CI, 1.19 to 4.82). In addition, duration of pesticide use equal and greater to 20 years was associated with lung cancer risk (HR, 2.43; 95% CI, 1.05 to 5.65). Average frequency of pesticide use was not associated with the risk of lung cancer. In contrast, there were no statistical associations between occupational pesticide exposure-related variables and lung cancer risk for women. The findings from time-lag analyses after excluding subjects with a follow-up period of equal or less than 3 years were also not much different from primary analyses (S1 Table).
All matched samples tended toward the characteristics of the unmatched data. Fig. 2 compares the covariate balance values for matching, stratification, and IPW by using the absolute standardized difference between the groups that did and did not use pesticides. Without the use of PS methods, the covariate balance was insufficient for almost all variables. Matching and stratification produced an excellent balance for all variables. IPTW achieved satisfactory covariate balance, except in year of cohort entry, the study area, owing to borderline cases. Due to the lack of covariate balance, the results of the analyses that did not use PS methods may be considered unreliable.
A comparison of the associations between occupational exposure to pesticides and lung cancer risk assessed by PS-based methods is shown in Table 3. The HRs ranged from 1.65 (1.04, 2.64) (regression adjusted for PS, as continuous term) to 2.84 (1.81, 4.46) (stratification by 5 strata of the PS).
DiscussionTo the best of our knowledge, this is the first report in which occupational exposure to pesticides has been found to be associated with lung cancer incidence using various PS methods or conventional covariate adjustment. Using data on the history of exposure to pesticides and lung cancer in the KNCCCS, we found estimated HRs (CI) ranging from 1.82 (1.11–2.98) (multiple Cox proportional hazards regression model) to 2.84 (1.81–4.46) for the stratification by five PS strata and PS-based methods yielded stronger associations than those obtained from the Cox proportional hazards regression model. There are advantages and disadvantages to PSM. PSM provides reliable results with excellent covariate balance, stratification retains data from all study subjects, and IPTW is easy to implement. In contrast, PSM leads to the exclusion of unmatched cases to ensure the best matching, stratification performs poorly when there are few outcome events, and IPTW may provide imprecise estimates of the treatment effect [15]. The meaningful findings of this study are consistent, regardless of whether PSM was used. Thus, this study provides strong evidence for the relationship between occupational exposure to pesticides and lung cancer risk.
Several epidemiological studies have found associations between exposure to pesticides and lung cancer [8–10]. In a cumulative meta-analysis of several cohort studies, the pooled relative risk estimate for lung cancer due to exposure to pesticides was 1.18 (standardized mortality ratio, 1.03 to 1.35; p=0.014) [9]. In a small, nested case-control study of structural pesticide applicators in Florida, Pesatori et al. [8] observed suggestive positive associations between exposure to pesticides and lung cancer (odds ratio, 2.4; 95% CI, 1.0 to 5.9). The AHS reported positive associations between select pesticides and the risk of lung cancer. Similar results were replicated in later studies with the AHS cohort [10]. The AGRIculture and CANcer (AGRICAN) cohort study of French farmers also suggested associations between the risk of small-cell lung cancer and exposure to pesticides (HR, 2.38; 95% CI, 1.07 to 5.28) [11]. These significant trends in the risk of lung cancer were also observed in our adjusted results. However, a number of studies have shown nonsignificant associations [21–23] or negative relationships [24–27] between occupational exposure to pesticides and lung cancer. Although several studies controlled for some important risk factors for lung cancer, such as indoor/outdoor air pollutants [28,29], lifestyle and psychosocial factors [30], and genetic predisposition [31], when assessing the association between pesticide exposure and lung cancer, such factors have not been routinely taken into account. In addition, impurities or promoting agents in pesticide formulations, such as dioxin and dioxin-like contaminants in phenoxy herbicides [5,32], might have contributed to the significant association found between exposure to some pesticides and lung cancer [5,8,32].
We found an association between the use of pesticides and lung cancer in the present analysis, although we did not obtain detailed exposure information for each type of pesticide. In vivo studies have shown that pesticides induce biochemical changes, leading to the development of tumor cells in the lung [33]. Since aryl hydrocarbon receptor activation mediates the tumorigenicity of dioxin-like compounds, microRNA expression induced by pesticides regulates lung cancer promotion at a wide range of workplace sites [34]. There is also experimental mechanistic evidence that pesticides such as chlorpyrifos and carbofuran can induce oxidative stress and oxidative DNA damage [35,36] and that pesticides may be genotoxic [37].
This study has several limitations. The number of lung cancer patients who had been exposed to pesticides was small, which negatively affected the precision of the results and our ability to evaluate risk stratified by the histologic type of lung cancer. Second, we did not have detailed exposure information, including years of use, applications per year, and applications in a lifetime, for each pesticide. Third, we could not fully control the effect of the study area as a confounding variable, although it was included multiple models. We attempted to consider stratification analysis by study area. However, the sample size according to study area was insufficient to obtain statistical power for stratification analysis. Forth, the selection biases that are present in most observational studies may impair causal inference in this study. To improve the ability to detect a causal relationship between exposure to pesticides and lung cancer incidence, we performed PS-based analyses in a community-based cohort. A major advantage of using PS-based methods in observational studies is that selection biases can be minimized by balancing nonrandomized individuals’ data to reach the level of causality determined in randomized controlled trials. Finally, there is the possibility of information bias due to the use of a self-reported questionnaire. However, the relationship between exposure to pesticides and lung cancer incidence was consistent across several PS methods, including stratification, matching, IPW, and use of the PS as a covariate in a logistic regression model, and the relationship revealed was stronger than that demonstrated in previous studies.
We found that occupational exposure to pesticides is associated with lung cancer risk. Inadequate control of the effects of covariates may have masked these effects in earlier studies. Although this study did not measure specific pesticides, we did observe a strong effect of occupational exposure to pesticides on the development of lung cancer. Further evaluations using PS methods of the associations between specific exposure to pesticides and a detailed history of exposure to pesticides and lung cancer risk are needed.
Supplementary InformationSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study protocols were approved by the Institutional Review Board of the National Cancer Center of Korea (IRB number: NCC2016-0300; NCC2017-0217). Signed written informed consent was obtained from all participants. AcknowledgmentsWe appreciate our collaborators at the public health centers of Haman-gun, Changwon-si, Chungju-si, and Chuncheon-si and the Health Center and County Hospital of Sancheong-gun for their support with regard to the implementation of the field surveys and data collection.
This study has been financially supported by the National Cancer Center of Korea (grant number: NCC-1710240-2).
Fig. 2Comparison of the standardized mean differences by covariates with different propensity score-based methods according to occupational exposure to pesticides. Crude, whole dataset; Matched, 1:1 matched dataset; Strata5, dataset stratified into five strata; IPTW, inverse probability-of-treatment weighting dataset. This plot shows the standardized mean differences between study subjects who had been occupationally exposed to pesticides and those who had not; a value > 0.1 indicates the imbalance of a covariate. 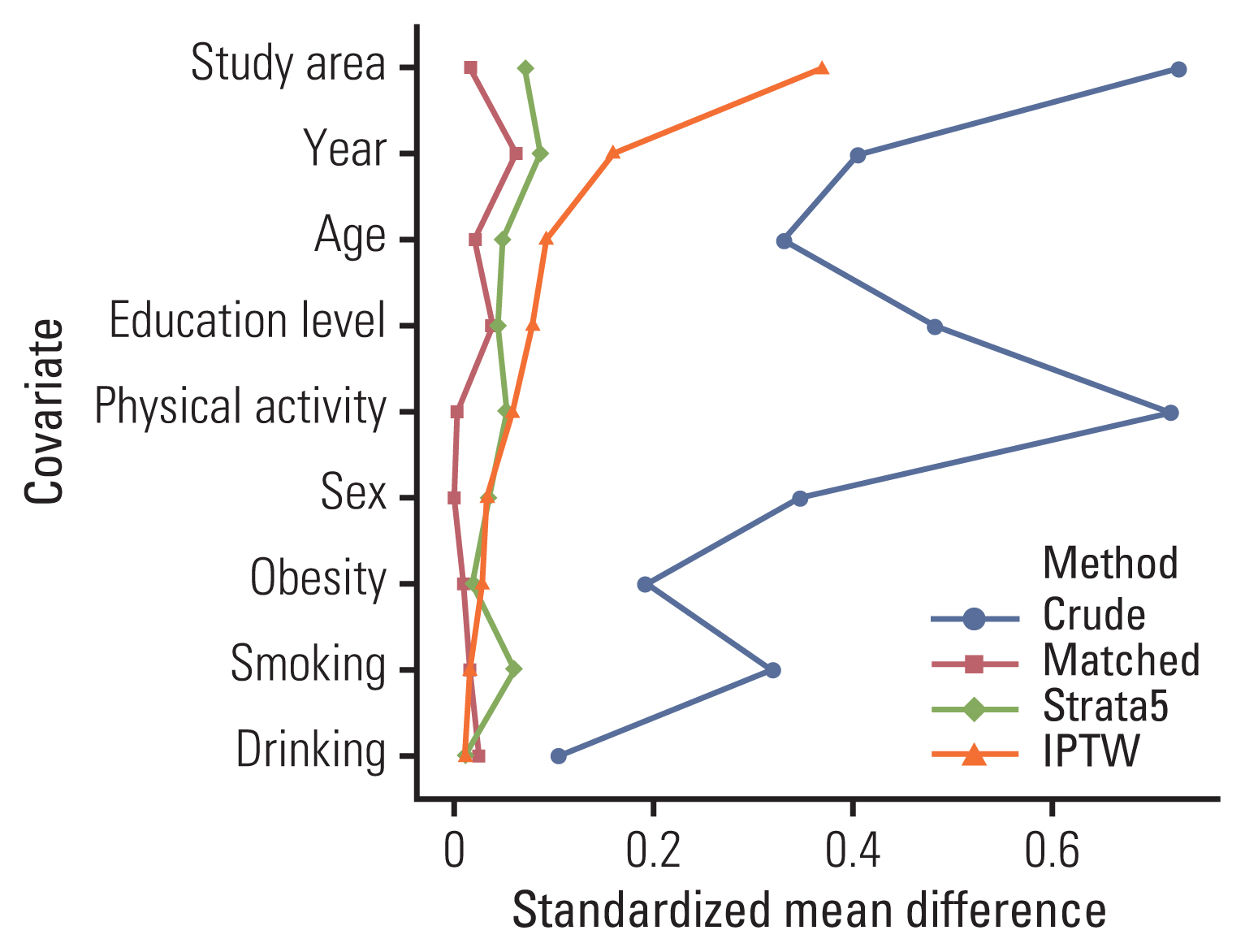
Table 1Baseline characteristics of the study participants Table 2Hazard ratios for lung cancer incidence according to history of occupational pesticide exposure, and smoking status Table 3Association between occupational exposure to pesticides and lung cancer incidence determined by PS-based methods References1. van der Plaat DA, de Jong K, de Vries M, van Diemen CC, Nedeljkovic I, Amin N, et al. Occupational exposure to pesticides is associated with differential DNA methylation. Occup Environ Med. 2018;75:427–35.
2. Statistics KoreaAnnual report on the cause of death statistics. Daejeon: Statistics Korea; 2016.
3. World Health OrganizationWHO handbook on indoor radon: a public health perspective. Geneva: World Health Organization; 2009.
4. Barthel E. Increased risk of lung cancer in pesticide-exposed male agricultural workers. J Toxicol Environ Health. 1981;8:1027–40.
5. Becher H, Flesch-Janys D, Kauppinen T, Kogevinas M, Steindorf K, Manz A, et al. Cancer mortality in German male workers exposed to phenoxy herbicides and dioxins. Cancer Causes Control. 1996;7:312–21.
6. Blair A, Dosemeci M, Heineman EF. Cancer and other causes of death among male and female farmers from twenty-three states. Am J Ind Med. 1993;23:729–42.
7. MacMahon B, Monson RR, Wang HH, Zheng TZ. A second follow-up of mortality in a cohort of pesticide applicators. J Occup Med. 1988;30:429–32.
8. Pesatori AC, Sontag JM, Lubin JH, Consonni D, Blair A. Cohort mortality and nested case-control study of lung cancer among structural pest control workers in Florida (United States). Cancer Causes Control. 1994;5:310–8.
9. Bonner MR, Freeman LE, Hoppin JA, Koutros S, Sandler DP, Lynch CF, et al. Occupational exposure to pesticides and the incidence of lung cancer in the agricultural health study. Environ Health Perspect. 2017;125:544–51.
10. Alavanja MC, Dosemeci M, Samanic C, Lubin J, Lynch CF, Knott C, et al. Pesticides and lung cancer risk in the agricultural health study cohort. Am J Epidemiol. 2004;160:876–85.
11. Boulanger M, Tual S, Lemarchand C, Guizard AV, Delafosse P, Marcotullio E, et al. Lung cancer risk and occupational exposures in crop farming: results from the AGRIculture and CANcer (AGRICAN) cohort. Occup Environ Med. 2018;75:776–85.
12. Jones RR, Barone-Adesi F, Koutros S, Lerro CC, Blair A, Lubin J, et al. Incidence of solid tumours among pesticide applicators exposed to the organophosphate insecticide diazinon in the Agricultural Health Study: an updated analysis. Occup Environ Med. 2015;72:496–503.
13. Silver SR, Bertke SJ, Hines CJ, Alavanja MC, Hoppin JA, Lubin JH, et al. Cancer incidence and metolachlor use in the Agricultural Health Study: an update. Int J Cancer. 2015;137:2630–43.
14. US Environmental Protection AgencyOffice of pesticide programs: list of chemicals evaluated for carcinogenic potential. Washington, DC: Environmental Protection Agency; 2002.
15. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69:345–57.
16. Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–8.
17. Oh JK, Lim MK, Yun EH, Choi MH, Hong ST, Chang SH, et al. Cohort profile: community-based prospective cohort from the National Cancer Center, Korea. Int J Epidemiol. 2017;46:e14.
18. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999–2001: first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–31.
19. Riaz SP, Horton M, Kang J, Mak V, Luchtenborg M, Moller H. Lung cancer incidence and survival in England: an analysis by socioeconomic deprivation and urbanization. J Thorac Oncol. 2011;6:2005–10.
20. Yoshida K, Bartel A, Chipman JJ, Bohn J, McGowan LD, Barrett M, et al. Create ‘Table 1’ to describe baseline characteristics with or without propensity score weights [Internet]. Vienna: The Comprehensive R Archive Network; 2020. [cited 2018 Apr 4]. Available from: https://cran.r-project.org/web/packages/tableone/tableone.pdf
21. Christensen CH, Platz EA, Andreotti G, Blair A, Hoppin JA, Koutros S, et al. Coumaphos exposure and incident cancer among male participants in the Agricultural Health Study (AHS). Environ Health Perspect. 2010;118:92–6.
22. Freeman LE, Rusiecki JA, Hoppin JA, Lubin JH, Koutros S, Andreotti G, et al. Atrazine and cancer incidence among pesticide applicators in the agricultural health study (1994–2007). Environ Health Perspect. 2011;119:1253–9.
23. Kang D, Park SK, Beane-Freeman L, Lynch CF, Knott CE, Sandler DP, et al. Cancer incidence among pesticide applicators exposed to trifluralin in the Agricultural Health Study. Environ Res. 2008;107:271–6.
24. Greenburg DL, Rusiecki J, Koutros S, Dosemeci M, Patel R, Hines CJ, et al. Cancer incidence among pesticide applicators exposed to captan in the Agricultural Health Study. Cancer Causes Control. 2008;19:1401–7.
25. Lynch SM, Mahajan R, Beane Freeman LE, Hoppin JA, Alavanja MC. Cancer incidence among pesticide applicators exposed to butylate in the Agricultural Health Study (AHS). Environ Res. 2009;109:860–8.
26. Mahajan R, Blair A, Lynch CF, Schroeder P, Hoppin JA, Sandler DP, et al. Fonofos exposure and cancer incidence in the agricultural health study. Environ Health Perspect. 2006;114:1838–42.
27. Rusiecki JA, Patel R, Koutros S, Beane-Freeman L, Landgren O, Bonner MR, et al. Cancer incidence among pesticide applicators exposed to permethrin in the Agricultural Health Study. Environ Health Perspect. 2009;117:581–6.
28. Enomoto M, Tierney WJ, Nozaki K. Risk of human health by particulate matter as a source of air pollution: comparison with tobacco smoking. J Toxicol Sci. 2008;33:251–67.
29. Samet JM, Eradze GR. Radon and lung cancer risk: taking stock at the millenium. Environ Health Perspect. 2000;108(Suppl 4):635–41.
31. Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408.
32. Kogevinas M, Becher H, Benn T, Bertazzi PA, Boffetta P, Bueno-de-Mesquita HB, et al. Cancer mortality in workers exposed to phenoxy herbicides, chlorophenols, and dioxins. An expanded and updated international cohort study. Am J Epidemiol. 1997;145:1061–75.
33. Walker NJ, Crockett PW, Nyska A, Brix AE, Jokinen MP, Sells DM, et al. Dose-additive carcinogenicity of a defined mixture of “dioxin-like compounds”. Environ Health Perspect. 2005;113:43–8.
34. Boffetta P, Mundt KA, Adami HO, Cole P, Mandel JS. TCDD and cancer: a critical review of epidemiologic studies. Crit Rev Toxicol. 2011;41:622–36.
35. Ojha A, Srivastava N. In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat Res Genet Toxicol Environ Mutagen. 2014;761:10–7.
|
|
||||||||||||||||||||||||||||||||||||||||||||||