AbstractPurposeRadiation-induced lymphopenia is associated with worse outcomes in solid tumors. We assessed the impact of interleukin-7 (IL-7), a key cytokine in lymphocyte homeostasis, on radiation-induced lymphopenia.
Materials and MethodsA post-hoc analysis was performed in a prospective cohort of 98 patients with hepatocellular carcinoma who were treated with radiotherapy in 2016–2018. Blood IL-7 levels were assayed before and at the end of radiotherapy. Acute severe lymphopenia (ASL) was defined as a total lymphocyte count of < 200/μL during radiotherapy. Cox and logistic regression analyses were performed to identify predictors of survival and ASL development, respectively.
ResultsPatients with ASL (n=41) had significantly poorer overall survival than those without (12.0 months vs. 25.3 months, p=0.001). Patients with lymphocyte recovery showed significantly longer overall survival than those without (21.8 months vs. 10.3 months, p=0.042). ASL was an independent predictor of poor survival (hazard ratio, 2.07; p=0.015). Patients with ASL had significantly lower pre-radiotherapy IL-7 levels (2.07 pg/mL vs. 3.01 pg/mL, p=0.010). A high pre-radiotherapy IL-7 level was an independent predictor of a reduced risk of ASL development (hazard ratio, 0.40; p=0.004). IL-7 levels reflected a feedback response to ASL, with a higher ΔIL-7 in patients with ASL and a lower ΔIL-7 in those without ASL (0.48 pg/mL vs. −0.66 pg/mL, p < 0.001). Post-radiotherapy IL-7 levels were significantly positively correlated with the total lymphocyte counts at 2 months.
IntroductionLymphocytes are critical elements that mediate antitumor immune responses. A decrease in the total lymphocyte count and lymphocyte infiltration in pathological specimens is associated with poor overall survival and progression-free survival [1–6]. Both CD8+ T-cells and natural killer cells are critical mediators of the antitumor response owing to their ability to produce interferon-γ and to directly kill the target cells [7,8]. Moreover, T lymphocytes contribute significantly to the efficacy of anticancer immunotherapy, including immune checkpoint inhibitors [9].
Lymphocytes and their precursors are very sensitive to ionizing radiation [10]. The lethal doses required to reduce the surviving fraction of lymphocytes by 50% and 90% are 2 Gy and 3 Gy, respectively [11]. Although radiotherapy can induce lymphopenia by damaging the bone marrow, local radiotherapy to non-marrow organs (e.g., the liver, brain, esophagus, rectum, and pancreas) can also cause systemic lymphopenia due to irradiation of the circulating blood during the course of conventional fractionated radiotherapy [1–6]. Therefore, novel strategies are urgently needed to preserve the total lymphocyte count during radiotherapy. Identifying factors associated with radiation-induced lymphopenia will be the first step in overcoming the rapid depletion of lymphocytes during radiotherapy. However, little is known about the physiological response to and predictors of radiation-induced lymphopenia in patients with solid tumors.
Interleukin (IL)-7, IL-15, and IL-2 are cytokines that possess a common γ-chain, which are critical for regulating lymphocyte homeostasis [12]. Recently, IL-7 has received increasing attention because its recombinant form was safely administered to cancer patients [13]. Moreover, the use of IL-7 for overcoming lymphopenia and enhancing the therapeutic effect in cancer patients is being actively explored in several clinical trials [10,14]. In a lymphopenic condition, the circulating levels of IL-7 increase, thereby promoting lymphocyte development in the thymus and maintaining the homeostasis of peripheral naive and memory T cells [15].
However, few studies have evaluated the effect of IL-7 on radiation-induced lymphopenia, which is characterized by the rapid depletion of lymphocytes during radiotherapy. Therefore, we aimed to identify the role of IL-7 in radiation-induced lymphopenia and to determine the risk factors and prognostic significance of radiation-induced lymphopenia using a prospective patient cohort.
Materials and Methods1. Study populationWe performed a post-hoc analysis in a prospective cohort of patients with hepatocellular carcinoma who were treated with radiotherapy between July 2016 and October 2018. The cohort was originally established to determine serum biomarkers for predicting treatment outcomes after radiotherapy in patients with locally advanced hepatocellular carcinoma.
The eligibility criteria were as follows: (1) unresectable primary hepatocellular carcinoma treated with radiotherapy, (2) age > 20 years, (3) Eastern Cooperative Oncology Group performance status of 0–2, (4) Child-Pugh class A/B, (5) normal liver volume > 1,000 cm3, (6) distance between the liver and organs at risk ≥ 0.5 cm, (7) adequate liver function (aspartate aminotransferase/alanine aminotransferase < 5 times the upper limit of normal, total bilirubin < 3.0 mg/dL, albumin > 2.5 g/dL, and normal prothrombin time/partial thromboplastin time), (8) adequate renal function (creatinine < 1.8 mg/dL or creatinine clearance rate > 50 mL/min), and (9) bone marrow reserve (absolute neutrophil count ≥ 1,500/mm3, platelet count ≥ 50,000/mm3; and hemoglobin level > 9 g/dL). Patients who received stereotactic body radiotherapy or previous radiotherapy to the upper abdomen were excluded. Ninety-nine patients were enrolled. One patient who had received radiotherapy to the head and neck for a separate malignancy was excluded, because previous radiotherapy may have affected the total lymphocyte count. In total, 98 patients were analyzed.
Conventional radiotherapy was administered to all patients, most of whom received the same dose/fractionation. Ninety-one patients (92.9%) received 100 Gy in 25 fractions to the gross tumor volume and 60 Gy in 25 fractions to the planning target volume using the simultaneous integrated boost technique. Sixty-six patients received combination therapy: concurrent hepatic arterial infusion chemotherapy (500 mg/m2/day of 5-fluorouracil) during the first and fifth week of radiotherapy (n=63) [16] or daily tegafur-uracil (n=3). The remaining 33 patients received radiotherapy only.
2. Assessment of total lymphocyte countPeripheral blood counts were measured weekly during radiotherapy and then every 1–3 months after radiotherapy for 1 year. Total lymphocyte count data were classified according to the number of months from the initiation of radiotherapy. Lymphopenia was graded using the Common Terminology Criteria for Adverse Events ver. 4.0 [17]. A total lymphocyte count from the lower limit of normal to 800 cells/μL was considered as grade 1; 500–800 cells/μL, grade 2; 200–500 cells/μL, grade 3; and < 200 cells/μL, grade 4. Acute severe lymphopenia (ASL) was defined as a total lymphocyte count of < 200 cells/μL during radiotherapy (i.e., grade 4 lymphopenia).
3. IL-7 cytokine assaysBlood samples (5–10 mL) were collected in vacutainer tubes (BD, Franklin Lakes, NJ) containing ethylenediaminetetraacetic acid before and at the last day of radiotherapy. The blood samples were centrifuged at 3,000 rpm for 10 minutes at 4°C to separate the buffy coat and plasma. Additional centrifugation for 10 minutes was performed to obtain cell-free plasma. The plasma aliquots were collected within 1–2 hours of blood sampling and frozen at −80°C until further processing. Plasma IL-7 concentrations were determined using a magnetic bead-based 6-plex immunoassay (customized Procartaplex, Thermo Scientific, Waltham, MA). Briefly, standards at different concentrations and patient plasma samples were mixed with antibody-linked polystyrene beads in 96-well filter-bottom plates and incubated at room temperature for 2 hours on an orbital shaker at 500 rpm. The plate was inserted into a magnetic plate washer and washed twice, followed by incubation with a biotinylated detection antibody mixture for 30 minutes at room temperature on an orbital shaker at 500 rpm. The samples were washed twice and resuspended in streptavidin-phycoerythrin. After incubation for 30 minutes at room temperature, two additional washes were performed, and the samples were resuspended in reading buffer. Each sample was measured in duplicate along with the standards (seven-point dilutions) and buffer control. Plates were read on the Luminex MAGPIX System (Merck Millipore, Burlington, MA) for quantitative analysis. The median fluorescence intensity of the analytes was detected using the flow-based MAGPIX System (Merck Millipore). Cytokine concentrations were calculated using Luminex xPONENT 4.2 software. A five-parameter model was used to calculate the final concentrations via interpolation.
4. Statistical analysisTwo-sided unpaired or paired t tests were used for statistical analysis. Survival rates were calculated from the time of the initiation of radiotherapy using the Kaplan-Meier method and compared using the log-rank test. Cox regression and binary logistic regression modeling were performed to identify predictors of overall survival and ASL development, respectively; backward stepwise selection of variables was performed. The predictive value of pre-radiotherapy IL-7 levels for ASL development was assessed using receiver operating characteristic curve analysis. The optimal cut-off value was determined using Youden’s index. Statistical analyses were performed using SPSS ver. 25.0 (IBM Corp., Armonk, NY) and Prism 7 (GraphPad Software, La Jolla, CA). Statistical significance was defined as p < 0.05.
Results1. Patient characteristicsThe patient, tumor, and treatment characteristics are summarized in Table 1. The median tumor size was 6.5 cm (range, 1.3 to 21.0 cm), and the median planning target volume was 594 cm3 (range, 37 to 5,156 cm3). Most patients (87.8%) had Child-Pugh class A disease. Approximately half of the patients had Barcelona Clinic Liver Cancer stage C disease. The majority of patients had hepatitis B virus infection (73.5%). No inflammatory or autoimmune diseases other than hepatitis were noted.
2. Changes in total lymphocyte count over timeThe mean total lymphocyte count decreased to 27% at 1 month after the initiation of radiotherapy, recovered to 104% of the baseline level at 2 months, and then remained low at 60%–70% of the initial level from 3–6 months (Fig. 1A). When compared to the mean baseline total lymphocyte count (1,122 cells/μL), the mean total lymphocyte count was significantly reduced at 1 month after the initiation of radiotherapy (305 cells/μL) and at 3 (787 cells/μL), 4 (775 cells/μL), 5 (686 cells/μL), and 6 months (752 cells/μL) (Bonferroni-adjusted p < 0.001). The mean total lymphocyte count at 2 months was not significantly different from that at baseline. During radiotherapy, 41 of 98 patients (41.8%) developed ASL.
3. Effects of the development of and recovery from radiation-induced lymphopenia on survival outcomesThe median follow-up was 20.5 months (range, 2.8 to 42.2 months). Patients with ASL had poorer overall survival (2-year overall survival rate, 30.3% vs. 50.2%; median, 12.0 months vs. 25.3 months; p=0.001) and progression-free survival (2-year progression-free survival rate, 8.1% vs. 20.4%; median, 6.4 months vs. 10.2 months; p=0.066) than those without. Among patients with ASL, those who recovered (total lymphocyte count > 1,000 cells/μL at 2 months, n=20) had significantly longer overall survival than those who did not (n=21) (2-year overall survival rate, 41.7% vs. 19.0%; median, 21.8 months vs. 10.3 months; p=0.042). Patients who recovered from ASL had an overall survival similar to that of those who initially had no ASL (median, 21.8 months vs. 25.3 months; p=0.158), while patients who did not recover had the poorest overall survival (median, 10.3 months) (Fig. 1B–E).
In multivariable analysis, Child–Pugh class B disease (hazard ratio [HR], 2.27, 95% confidence interval [CI], 1.15 to 4.49; p=0.018), the Union for International Cancer Control stage III/IV (HR, 1.78; 95% CI, 1.27 to 2.50; p=0.001), and ASL development (HR, 2.03; 95% CI, 1.24 to 3.31; p=0.005) were significant predictors of poor overall survival (Table 2).
4. Effects of IL-7 on the development of and recovery from radiation-induced lymphopeniaThe median pre- and post-radiotherapy IL-7 levels for all patients were 2.22 pg/mL (range, 0.24 to 9.79 pg/mL) and 2.20 pg/mL (range, 0.20 to 7.33 pg/mL), respectively. Pre-radiotherapy IL-7 levels were significantly lower in patients who developed ASL than in those who did not (mean, 2.07 pg/mL vs. 3.01 pg/mL; p=0.010) (Fig. 2A). Pre-radiotherapy IL-7 levels were significantly positively correlated with the total lymphocyte count nadir during radiotherapy (Fig. 2C).
Differences in IL-7 levels before and after radiotherapy (post-radiotherapy minus pre-radiotherapy IL-7 levels [ΔIL-7]) were significantly higher in patients with ASL than in those without (mean, 0.48 pg/mL vs. −0.66 pg/mL; p < 0.001). The mean IL-7 level was significantly elevated after radiotherapy in patients with ASL (pre-radiotherapy vs. post-radiotherapy IL-7 levels, 2.07 pg/mL vs. 2.55 pg/mL; p=0.029), whereas it was significantly reduced in patients without ASL (3.01 pg/mL vs. 2.35 pg/mL, p=0.005) (Fig. 2B). The ΔIL-7 was significantly negatively correlated with the total lymphocyte count nadir during radiotherapy (Fig. 2D). The total lymphocyte count at 2 months after the initiation of radiotherapy was significantly positively correlated with post-radiotherapy IL-7 levels (Fig. 2E).
In multivariable analysis, grade ≥ 1 lymphopenia at baseline (HR, 29.81; 95% CI, 6.54 to 135.82; p < 0.001) and a large planning target volume (per 100 cm3 increase; HR, 1.23; 95% CI, 1.11 to 1.36; p < 0.001) were significantly associated with an increase in the incidence of ASL, while elevated pre-radiotherapy IL-7 levels were significantly associated with a decrease in the incidence of ASL (per 1 pg/mL increase; HR, 0.39; 95% CI, 0.22 to 0.69; p=0.001). Hepatitis B virus infection showed marginal significance (HR, 7.26; 95% CI, 0.98 to 53.79; p=0.052) (Table 3). In a separate multivariable analysis including the mean liver dose instead of the planning target volume, pre-radiotherapy IL-7 levels were an independent predictor of ASL development as well, along with grade ≥ 1 lymphopenia at baseline and mean liver dose (S1 Table).
Since pre-radiotherapy IL-7 levels were an independent predictor of ASL development, we classified patients into high and low pre-radiotherapy IL-7 groups using a cut-off value of 2.495 pg/mL (Table 4). The characteristics, except for Child-Pugh class and planning target volume, did not differ significantly between the groups. Twelve of 45 patients (26.7%) in the high pre-radiotherapy IL-7 group and 29 of 53 patients (54.7%) in the low pre-radiotherapy IL-7 group developed ASL (p=0.005).
The toxicity profile is shown in S2 Table. Neither pre-nor post-radiotherapy IL-7 levels differed significantly according to the presence of grade ≥ 2 gastrointestinal or liver toxicity.
DiscussionWe confirmed the previous findings that peripheral local radiotherapy can induce systemic lymphopenia and that radiation-induced lymphopenia can have detrimental effects on survival outcomes [1–6]. Most studies reported these findings using retrospective data. Our findings are meaningful because we obtained these results from a prospective cohort using a well-controlled blood test protocol and treatment schedule. We also suggest the role of IL-7 in radiation- induced lymphopenia based on our findings.
Emerging evidence suggests that radiation-induced lymphopenia has detrimental effects on the pathological responses and survival of patients with various types of cancer [1–6]. Since preserving the optimal lymphocyte count in the blood may be essential in achieving successful treatment outcomes, novel strategies to prevent and reverse radiation-induced lymphopenia are urgently needed. To develop these strategies, risk factors for radiation-induced lymphopenia need to be identified first. Previous studies have reported various radiotherapy-related risk factors, such as photon- vs. proton-based radiotherapy [18], brain volume receiving 25 Gy [19], and conventional vs. stereotactic body radiotherapy [4,20]. A previous mathematical model demonstrated that the chance of delivering radiation to circulating blood increases as the planning target volume and the number of fractions increase [21]. In a previous study, we retrospectively evaluated 920 patients with hepatocellular carcinoma who received radiotherapy. We found that a low baseline total lymphocyte count, large planning target volume, and multiple fractionations were risk factors for radiation-induced lymphopenia [5]. In the current study involving an independent prospective cohort, a low baseline total lymphocyte count and large planning target volume were also risk factors for ASL development, confirming our previous findings. Since most patients received the same dose fractionation regimen, we were unable to determine the influence of dose fractionation.
In addition to previously known predictors of radiation-induced lymphopenia, this study also identified an association between serum IL-7 levels and radiation-induced lymphopenia. To our knowledge, this study is one of the first to identify such an association. IL-7 induces lymphocyte proliferation and differentiation by activating various molecular pathways [15]. Changes in IL-7 levels precede changes in lymphocyte counts. Consistent with this, in our study, pre-radiotherapy IL-7 levels were significantly positively correlated with the total lymphocyte count nadir during radiotherapy and were an independent predictor of a reduced risk of ASL development. Considering the clinical relevance, we focused on the analysis of pre-radiotherapy IL-7 levels.
When patients were divided according to pre-radiotherapy IL-7 levels (Table 4), the high pre-radiotherapy IL-7 group had a significantly lower incidence of ASL, even though this group had a larger planning target volume. Considering planning target volume as a risk factor for ASL development, as shown in other studies [2,5,21], as well as in the current study, the high pre-radiotherapy IL-7 group was expected to have a higher incidence of ASL. However, it did not. Therefore, we assumed that although a large planning target volume was associated with an increased risk of ASL development, such a risk may be mitigated by a high pre-radiotherapy IL-7 level.
Furthermore, we observed a complementary feedback response of IL-7 on changes in total lymphocyte count during radiotherapy. The ΔIL-7 was negatively associated with total lymphocyte count during radiotherapy. The ΔIL-7 had a positive value among patients with ASL and a negative value among those without. Changes in serum IL-7 levels are known to reflect changes in the population of T-cells that consume cytokines [22]. Under lymphopenic conditions, T-cells encounter abundant IL-7, leading to homeostatic proliferation [23,24]. In contrast, when T-cells over proliferate, and patients become non-lymphopenic, IL-7 levels are reduced due to over consumption, leading to T cell death. Our findings are contrary to those of Ellsworth et al. [25]. In their study, patients with high-grade glioma treated with concurrent chemoradiotherapy showed lymphocyte depletion, but no increase in IL-7 levels. However, in addition to the small number of patients (n=11) in that study, IL-7 levels were not stratified according to the presence of lymphopenia, unlike in the current study. These discrepancies may have led to the different interpretations about the role of IL-7 in radiation-induced lymphopenia.
Our data showed that ASL was an independent predictor of poor overall survival, confirming previous findings [1–6]. There were no direct associations between pre- or post-radiotherapy IL-7 levels and overall survival. Therefore, we assumed that high IL-7 levels may indirectly influence survival by means of total lymphocyte count preservation. The administration of exogenous IL-7 before radiotherapy can be a good option to prevent a decline in total lymphocyte count during radiotherapy, particularly in patients with a high risk of ASL due to a large planning target volume, multiple fractionations, or a low baseline total lymphocyte count. The effect of exogenous IL-7 on increasing the number of T lymphocytes has been demonstrated in earlier studies of human immunodeficiency virus-infected patients and patients with melanoma or sarcoma [13,26]. Currently, a clinical study is being conducted to assess the ability of exogenous IL-7 to restore the total lymphocyte count after radiotherapy [27]. The finding that elevated IL-7 levels after radiotherapy were associated with lymphocyte recovery suggests that IL-7 can also be administered after radiotherapy for lymphocyte recovery; this is important because, according to our results, ASL recovery mitigated poor survival outcomes. A retrospective study of 167 patients with solid tumors treated with immune checkpoint inhibitors also showed that rapid recovery of the total lymphocyte count is associated with improved outcomes [28]. In some patients, IL-7 is not secreted despite the low total lymphocyte count during radiotherapy, as can be observed in the lower left part of Fig. 2D. This may be attributed to the lack of feedback mechanisms. Accordingly, those patients may be candidates who may benefit from exogenous IL-7 administration after radiotherapy.
A limitation of our study is that the type of tumor was confined to hepatocellular carcinoma. However, compared with other types of tumors, hepatocellular carcinoma has a unique feature with regard to radiotherapy-related lymphopenia in that the liver harbors a very rich blood supply and the tumor itself is hypervascular, leading to a greater amount of blood being exposed to radiation, which can maximize the radiation effect and cause lymphopenia [5]. Another limitation, considering the dynamic changes in IL-7 levels, is that only two samples were obtained. Further studies are needed to understand the relationship between the dynamic changes in IL-7 levels and radiation-induced lymphopenia. Finally, immune reactions during radiotherapy may be related to different types of cytokines and immune cells. However, in this study, we only focused on IL-7 levels and total lymphocyte counts [29]. Our results should be interpreted with caution, and the relationship between various cytokines or immune cells and radiation-induced lymphopenia should be addressed in future studies.
In conclusion, IL-7 levels are associated with the prevention of and recovery from radiation-induced lymphopenia. Radiation-induced lymphopenia and no recovery from it were associated with poor survival. High IL-7 levels may indirectly influence survival by means of total lymphocyte count preservation. Therefore, to overcome radiation-induced lymphopenia and to enhance the therapeutic effect of radiotherapy, a novel strategy using the cytokine IL-7 (e.g., the administration of exogenous IL-7) may be considered.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The protocol and any amendments to the methodology were approved by the Institutional Review Board (approval number: 4-2017-0093) of Yonsei University prior to the initiation of the study. Research was conducted in accordance with the Declaration of Helsinki. All patients provided informed consent before enrollment. AcknowledgmentsThis study was supported by the National Nuclear R&D Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (grant number: 2017070426), and Dong-A research fund (grant number: 2018-31-0904).
Fig. 1(A) Changes in the mean (standard deviation) total lymphocyte count (TLC) over time after the initiation of radiotherapy (RT). (B) Overall survival according to the presence of acute severe lymphopenia (ASL). (C) Progression-free survival according to the presence of ASL. (D, E) Overall survival according to the presence of ASL and the recovery from lymphopenia at 2 months after the initiation of RT. 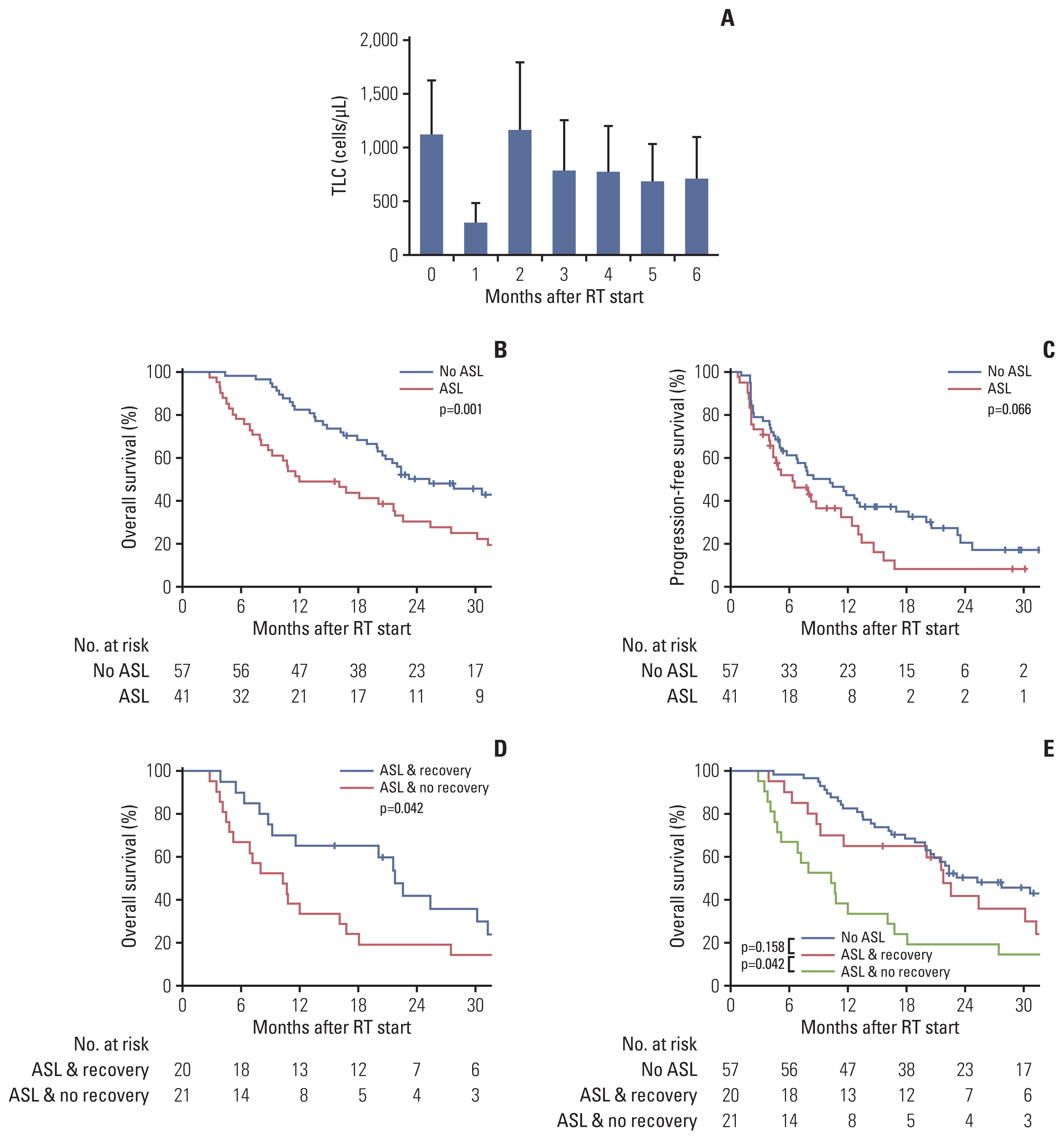
Fig. 2Relationship between interleukin-7 (IL-7) levels and radiation-induced lymphopenia. (A) Pre-radiotherapy (RT) IL-7 levels according to the presence of acute severe lymphopenia (ASL). (B) Changes in IL-7 levels (ΔIL-7) according to the presence of ASL and pre- and post-RT IL-7 levels in patients with and without ASL. (C) The correlation between pre-RT IL-7 levels and the total lymphocyte count (TLC) nadir during RT. (D) The correlation between the TLC nadir during RT and ΔIL-7. (E) The correlation between post-RT IL-7 levels and the TLC at 2 months after the initiation of RT. CI, confidence interval. *p < 0.05, **p < 0.01, ***p < 0.001. 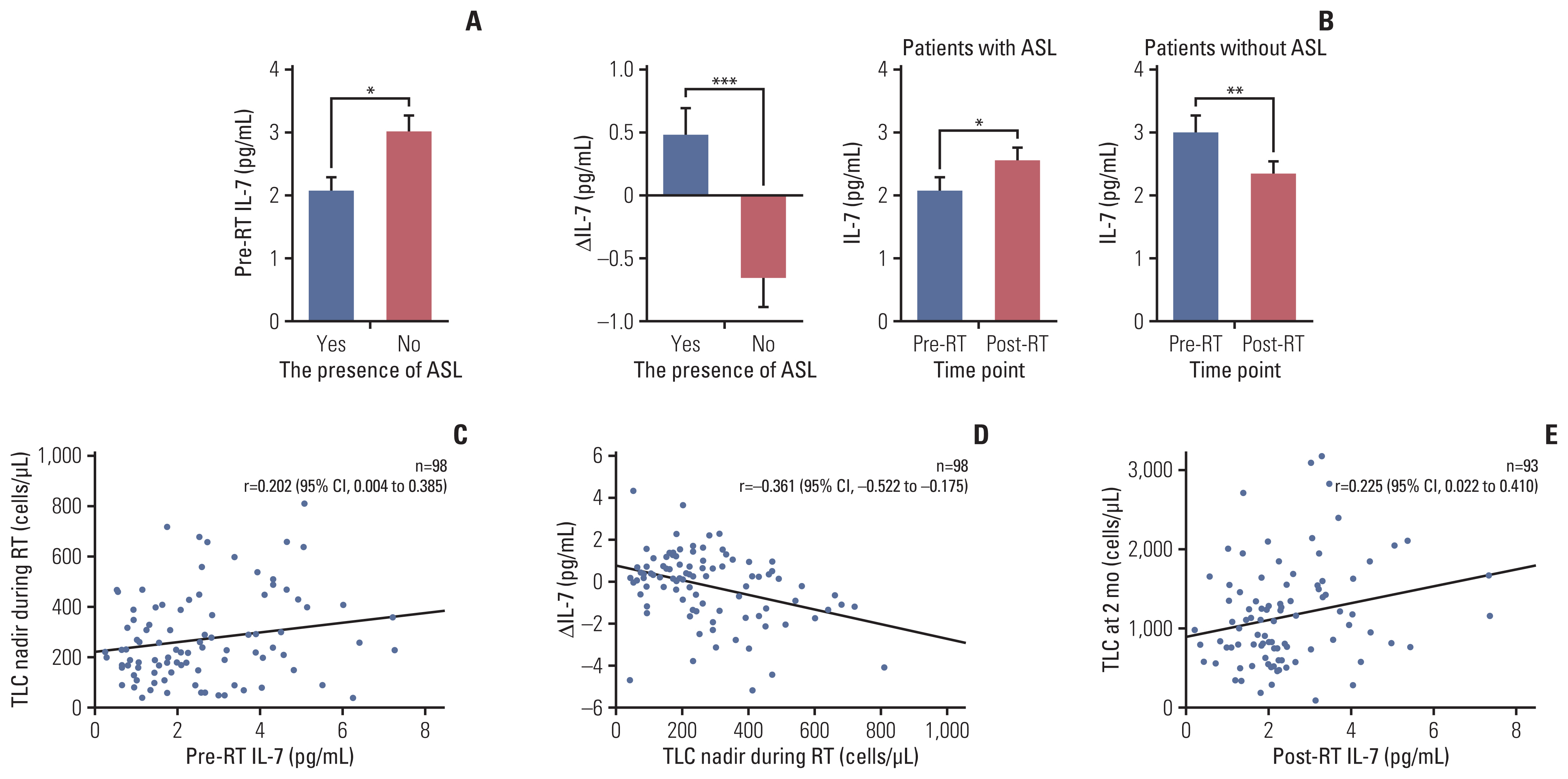
Table 1Patient characteristics Table 2Analysis of factors associated with overall survival
Table 3Analysis of factors affecting the development of acute severe lymphopeniaa)
Table 4Patient characteristics according to pre-radiotherapy IL-7 levels References1. Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015;13:1225–31.
2. Byun HK, Kim N, Yoon HI, Kang SG, Kim SH, Cho J, et al. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019;14:51.
3. Park S, Byun HK, Seong J. Irradiation-related lymphopenia for bone metastasis from hepatocellular carcinoma. Liver Cancer. 2019;8:468–79.
4. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;105:1065–73.
5. Byun HK, Kim N, Park S, Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. 2019;195:1007–17.
6. Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, et al. Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: Immunologic relevance. Radiother Oncol. 2019;131:52–9.
7. Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222:11–20.
8. Tsukumo SI, Yasutomo K. Regulation of CD8(+) T cells and antitumor immunity by Notch signaling. Front Immunol. 2018;9:101.
9. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81.
10. Button LN, DeWolf WC, Newburger PE, Jacobson MS, Kevy SV. The effects of irradiation on blood components. Transfusion. 1981;21:419–26.
11. Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123:224–7.
12. Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79.
13. Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–9.
14. Duane FK, McGale P, Teoh S, Mortimer C, Broggio J, Darby SC, et al. International variation in criteria for internal mammary chain radiotherapy. Clin Oncol (R Coll Radiol). 2019;31:453–61.
16. Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003.
17. Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008;26:5735–41.
18. Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128:154–60.
19. Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen DF, Fergus S, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92:1000–7.
20. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;94:571–9.
21. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–4.
22. Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54.
23. Hakim FT, Gress RE. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology. Eur J Immunol. 2005;35:3099–102.
24. Napolitano LA, Burt TD, Bacchetti P, Barron Y, French AL, Kovacs A, et al. Increased circulating interleukin-7 levels in HIV-1-infected women. J Acquir Immune Defic Syndr. 2005;40:581–4.
25. Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014;3:e27357.
26. Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300.
27. Study of the effect NT-I7 on CD4 counts in patients with high grade gliomas [Internet]. Bethesda, MD: National Library of Medicine; 2016. [cited 2020 Jan 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT02659800
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||