AbstractPurposeThis study was conducted to determine the effectiveness of immune checkpoint inhibitors (ICIs) in recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) after platinum-containing chemotherapy. We also identified clinical biomarkers which may be predictive of patient prognosis.
Materials and MethodsWe analyzed 125 patients with R/M HNSCC who received ICIs, retrospectively. Overall response rate (ORR) was the primary study outcome. Overall survival (OS) and progression-free survival (PFS) were the secondary study outcomes.
ResultsThe patients received anti–programmed cell death protein-1 (PD-1) (n=73, 58%), anti–programmed death-ligand 1 (PD-L1) (n=24, 19%), or a combination of anti–PD-1/PD-L1 and anti–cytotoxic T-lymphocyte antigen 4 (n=28, 22%). The median age was 57 years (range, 37 to 87). The location of the primary tumor was in the oral cavity in 28% of the cases, followed by oropharynx (27%), hypopharynx (20%), and larynx (12%). The ORR was 15% (19/125). With 12.3 months of median follow-up, median PFS was 2.7 months. Median OS was 10.8 months. A neutrophil-to-lymphocyte ratio (NLR) > 4 was significantly associated with poor response to ICIs (odds ratio, 0.30; p=0.022). A sum of the target lesions > 40 mm (hazard ratio [HR], 1.53; p=0.046] and a NLR > 4 (HR, 1.75; p=0.009) were considered to be predictive markers of short PFS. A poor performance status (HR, 4.79; p < 0.001), a sum of target lesions > 40 mm (HR, 1.93; p=0.025), and an NLR > 4 (HR, 3.36; p < 0.001) were the significant predictors for poor survival.
IntroductionSquamous cell carcinoma and its associated variants represent the major histologic subtype that account for 90% of all head and neck tumor cases [1]. The combination chemotherapy with platinum, fluorouracil, and cetuximab, known as EXTREME regimen, improves the survival of patients (hazard ratio (HR) for death 0.80, p=0.04) and is the standard treatment for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) [2]. However, there are limited treatments for patients with R/M HNSCC that are resistant to platinum-containing chemotherapy. As a result, prognosis is dismal, and the patients survive for about 3.0–6.7 months [3,4]. Two phase 3 clinical studies evaluating the efficacy of tyrosine kinase inhibitors in epidermal growth factor receptor with gefitinib and afatinib failed to demonstrate a survival benefit compared with methotrexate treatment, the standard second-line therapy [4,5].
The coupling of programmed cell death protein-1 (PD-1) expressed by T cells with programmed death-ligand 1 (PD-L1), expressed by tumor cells as a mechanism of tumor immune evasion, heads to the suppression of the T-cell anti-tumor responses. The inhibitory immune checkpoint pathway can be blocked by immune checkpoint inhibitors (ICIs) to reactivate antitumor immune activity [6].
Nivolumab and pembrolizumab, a class of anti-PD-1 monoclonal antibodies, have been approved for patients with R/M HNSCC who failed platinum-containing chemotherapy regardless of the results of biomarker test [7,8]. ICI monotherapy exhibits a response rate of only ~20% or less. To identify predictive biomarkers of the ICI response, several potential predictive and/or prognostic biomarkers have been evaluated in HNSCC patients, including expression of tumor PD-L1 status, tumor mutation burden, and immune-related gene expression signatures [9–11]. However, in the case of tumor PD-L1 expression, the standard protocols for staining and interpreting results have not yet been established for the various ICIs. For high tumor mutational burden, which is one of the most actively studied genetic signature associated with a favorable outcome of ICI therapy, the costliness, and long turnaround time of whole-exome sequencing are major obstacles that limit its clinical use [12]. The investigation of immune-related gene signatures that can be used to predict outcome of ICI therapy is ongoing, and further studies are necessary to confirm this approach [13].
This study is aiming at determining the real-world effectiveness of ICIs therapy in R/M HNSCC patients and identifying clinical biomarkers that can be useful for predicting patient prognosis.
Materials and Methods1. Patient cohortAll patients diagnosed with R/M HNSCC, then treated with ICIs between January 2013 and December 2018 at eleven participating hospitals were included and analyzed retrospectively. This study was eligible for patients who were over 18 years of age and pathologically confirmed R/M HNSCC that was resistant to curative intent treatments. In addition, patients had previously received platinum-containing chemotherapy in the context of primary or recurrent stage, acceptable complete blood counts, adequate liver and kidney function. The patients whose disease had progressed within 6 months after the end of platinum-based definitive concurrent chemoradiotherapy were allowed to enroll in this study. At least one measurable target was necessary according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1.
Patients with nasopharyngeal cancer, or who had received previous therapy targeting immune checkpoint pathways, were excluded. Human papillomavirus (HPV) testing was not obligatory for oropharyngeal cancer patients.
2. Treatment of ICIsThis outcome study included all eligible patients who had received any combination of PD-1, PD-L1, and/or cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors during routine practice retrospectively. When gathering information regarding ICIs used in each hospital, we did not collect the exact names, but collected the class of ICIs used to ensure the neutrality of the analyst. Patients were received until progressive disease or unacceptable toxicities.
3. Study outcomes and statistical analysisThe primary study outcome was the overall response rate (ORR) by the criteria of RECIST ver. 1.1. Secondary study outcomes included progression-free survival (PFS) and overall survival (OS).
To compare patient characteristics and study outcomes between groups, independent t tests for continuous variables and chi-square tests for categorical variables as appropriate. Logistic-regression models were used to assess the association between ICIs and tumor response, after adjusting for other factors. Multivariate Cox regression analysis was performed to determine predictors for increased survival. A p ≤ 0.05 was the significant threshold. All statistical testing was conducted using Stata ver. 13.0 software (Stata Corp LP, College Station, TX).
Results1. Patient characteristicsThe baseline patient demographics are summarized in Table 1. A total of 125 patients with R/M HNSCC received ICIs. The median patients’ age was 57 years (range, 33 to 87 years), and 82% were male. More than half of the patients (56%) were current or former smokers. The site of the primary tumor was the oral cavity in 28% of the patients, followed by oropharynx (27%), hypopharynx (20%), and larynx (12%). The front-line treatment for advanced HNSCC was surgery for 46% of the patients, followed by concurrent chemoradiotherapy in 21%. The median number of systemic therapy lines prior to ICI treatment was one (range, 0 to 4). Study population included 15 (12%) patients with zero line of systemic therapy, who received platinum-based concurrent chemoradiotherapy but the disease progressed within 6 months of chemoradiotherapy.
ICI treatments included a PD-1 inhibitor in 58% of the patients, PD-L1 inhibitor in 19%, and a combination of PD-1/PD-L1 and CTLA-4 inhibitors in 22%. The interval between cancer diagnosis and ICI initiation was median 17.1 months. Patients had received ICIs with a median of three cycles (range, 1 to 34). The median sum of the target lesions prior to ICI therapy was 42 mm (range, 10 to 118 mm). The characteristics of the ICIs treatments are tabulated in Table 2.
2. Study outcomesAmong the 125 patients, eight (6%) did not receive post-ICI imaging and were not evaluated. The ORR was 15% (19/125; 95% confidence interval [CI], 3.6 to 36.8): a complete response was found in 3% (4/125), and a partial response was found in 12% (15/125) (Fig. 1). For responders, the median duration of response was 9.7 months (95% CI, 5.8 to 16.3). Following the analysis of the response rate with respect to the primary tumor location, the ORR was 21% for oropharyngeal cancer and 13% for non-oropharyngeal cancers (p=0.770 by the chi-square test). The antitumor activity of the ICIs is tabulated in Table 3. Among various clinical characteristics, a neutrophil-to-lymphocyte ratio (NLR) > 4 (vs. ≤ 4) was predictive of ICIs response (S1 Table). After adjustment for covariates, the association between NLR > 4 and poor response of ICIs (odds ratio [OR], 0.30; 95% CI, 0.11 to 0.84; p=0.022) remained significant (Table 4).
The PFS was 2.7 months (95% CI, 2.2 to 3.5), and the median OS was 10.8 months (95% CI, 7.5 to 18.7) (Fig. 2A and B). When we compared the median OS by the primary tumor location, patients with oropharyngeal cancer exhibited most favorable survival (Fig. 2C). Of note, OS was significantly different between oropharyngeal and non-oropharyngeal cancer (HR, 0.46; 95% CI, 0.24 to 0.86; p=0.016) (Fig. 2D).
3. Univariate and multivariate analyses for progression and deathBy uni- and multivariate analyses, a sum of the target lesions > 40 mm (vs. ≤ 40 mm) (HR, 1.53 [1.01–2.33]; p=0.046) and a NLR > 4 (vs. ≤ 4) (HR, 1.75 [1.15–2.65]; p=0.009) were independent risk factors for short PFS (Table 4, S2 Table). In terms of OS, a poor performance status (Eastern Cooperative Oncology Group performance status 2–3) (vs. 0–1), a sum of target lesions > 40 mm (vs. ≤ 40 mm), and an NLR > 4 (vs. ≤ 4) were the significant predictors for poor survival (Table 4, S3 Table).
DiscussionThis real-world data for the effectiveness of ICIs therapy in R/M HNSCC patients following platinum-containing chemotherapy exhibited a 15% response rate, a PFS of 2.7 months and an OS of 10.8 months, which is quite consistent with previous studies [7,8].
Prior to the introduction of ICI for HNSCC therapeutics, prognosis was dismal with only 6 months or less median survival, especially in the context of progressive disease following platinum-based chemotherapy within 6 months [5,14]. Based on two well-designed randomized controlled trials, anti–PD-1 antibodies proved survival benefits compared with second-line systemic therapy including methotrexate, docetaxel, and cetuximab in patients with refractory R/M HNSCC [7,8]. In the CheckMate-141 study, the OS was significantly increased in patients receiving nivolumab compared with patients receiving standard second-line systemic therapy (median OS, 7.5 months vs. 5.1 months; HR, 0.70; p=0.01) [7]. The ORR was also higher in patients who were given nivolumab compared with patients who receiving standard therapy (13.3% vs. 5.8%, respectively). In the KEYNOTE-040 study, Cohen et al. [8] found that pembrolizumab demonstrated a clinically meaningful increase in OS (8.4 months) compared with standard treatment (6.9 months) (HR, 0.80; p=0.0161). Additionally, treatment with pembrolizumab turned to an ORR of 14.6%.
Interestingly, our data showed differences in the antitumor activities of ICIs that were dependent on the primary tumor location. The ORR (21%) for oropharyngeal cancers was higher than those for non-oropharyngeal cancers (13%). In contrast, when the study population was grouped into oropharyneal or non-oropharyneal cancer, a significant difference in OS was observed (HR, 0.46; 95% CI, 0.24 to 0.86; p=0.016) (Fig. 2D).
The effect of HPV infection on outcomes could not be assessed in our study, because the number of study patients tested for HPV status was low. Though the proportion of patients with an ORR were similar regardless of HPV infection in phase Ib (KEYNOTE-012) and phase II (KEYNOTE-055) studies with pembrolizumab, further studies on predictive roles of HPV status were warranted [9,15].
Of note, we found that a baseline sum of the target lesions < 40 mm was an independent risk factor for longer PFS and OS. The summation of the longest dimension in all measurable targets quantifies the baseline tumor scale. Recent studies showed that baseline tumor scale was the significant prognostic factor of ICI therapy in non-small cell lung cancer and malignant melanoma [16,17]. Joseph et al. [16] reported that the baseline tumor scale below the median value was an independent risk factor of survival in pembrolizumab-treated patients with advanced malignant melanoma. Katsurada et al. [17] reported that baseline tumor scale above the median value was a negative predictive and prognostic indicator for the response to ICIs in non-small cell lung cancer. Given that the baseline tumor scale reflects overall burden of tumor, a low tumor burden might be a simple and clinically useful indicator of better outcomes of ICI treatment in R/M HNSCC patients.
As a clinical biomarker, we also found that an NLR prior to ICI therapy is strongly and independently associated with patient outcome. Our study indicated that a high NLR > 4 (vs. ≤ 4) lead to a poor response to ICIs (OR, 0.30; 95% CI, 0.11 to 0.84; p=0.022), a worse PFS (HR, 1.75; 95% CI, 1.15 to 2.65; p=0.009) and OS (HR, 3.36; 95% CI, 1.74 to 6.49; p < 0.001). Systemic inflammation is related to overall prognosis in patients with solid tumors because inflammation affects the immune response to tumors [18–21]. Given that the NLR is an established marker of the overall immune response to stress stimuli, the prognostic use of the NLR in context of ICI therapy is quite speculative. A recent meta-analysis demonstrated that a high NLR is related to worse outcomes throughout different reports [22]. This is likely because an elevated NLR reflects increased levels of peripheral blood regulatory T cells, granulocytes, and myeloid-derived suppressor cells, which play an important role in a suppressive tumor microenvironment and tumor progression [23,24]. Considering that the NLR can be easily evaluated from a routine complete blood count, it could provide a simple and inexpensive test for the prediction of R/M HNSCC prognosis.
A few limitations should be noted. First, retrospective design of the work may lead to selection bias. The various ICIs used in this work also contributed to heterogeneity of the study drug and patient population. However, the data were obtained from 11 referral hospitals and relatively large number of patients were enrolled (n=125), which reflects the real-world situation in the Korean patient population. In an era of ICIs treatment, we usually pay special attention to immune-related adverse events (IRAE), because the presence of IRAEs is related to a longer duration of response and are the major causes of drug discontinuation [25]. However, our study could not collect IRAEs because the retrospective nature of our study may have caused an underestimation of patient-reported IRAEs. Additionally, we could not retrieve the data regarding expression of tumor PD-L1 status due to the difference in PD-L1 staining methods used and interpreting results. Given the absence of a conclusion regarding the PD-L1 expression status as a reliable biomarker in the treatment of HNSCC with ICIs, it would be better to wait for the results of upcoming prospective studies [26].
Taken together, this retrospective study suggests that ICIs are effective in R/M HNSCC patients following platinum-containing chemotherapy. Furthermore, two simple and inexpensive clinical biomarkers, low tumor burden (a sum of target lesions ≤ 40 mm) and a low NLR (≤ 4) may serve as predictors to recognize patients with R/M HNSCC who benefit from receiving ICIs.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement Study protocol was approved in each institutional review board. Written informed consents were waived because of the retrospective nature of this study (CRIS Registration Number, KCT0004258, 10 May 2019). Author Contributions Conceived and designed the analysis: Lee YG, Chang H, Keam B, Chun SH, Park J, Park KU, Shin SH, An HH, Lee KE, Lee KW, Kim HR, Kim SB, Ahn MJ, Hwang IG. Collected the data: Lee YG, Chang H, Keam B, Chun SH, Park J, Park KU, Shin SH, An HH, Lee KE, Lee KW, Kim HR, Kim SB, Ahn MJ, Hwang IG. Contributed data or analysis tools: Lee YG, Chang H. Performed the analysis: Lee YG, Chang H. Wrote the paper: Lee YG, Chang H, Keam B, Ahn MJ, Hwang IG. AcknowledgmentsThis study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (HA16C0015) (1720150).
Fig. 1Efficacy of immune checkpoint inhibitors based on Response Evaluation Criteria in Solid Tumors ver. 1.1 by investigator assessment. The data shown represents the maximum percentage change from baseline in the sum of the longest diameter of the largest lesions (n=110). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease. 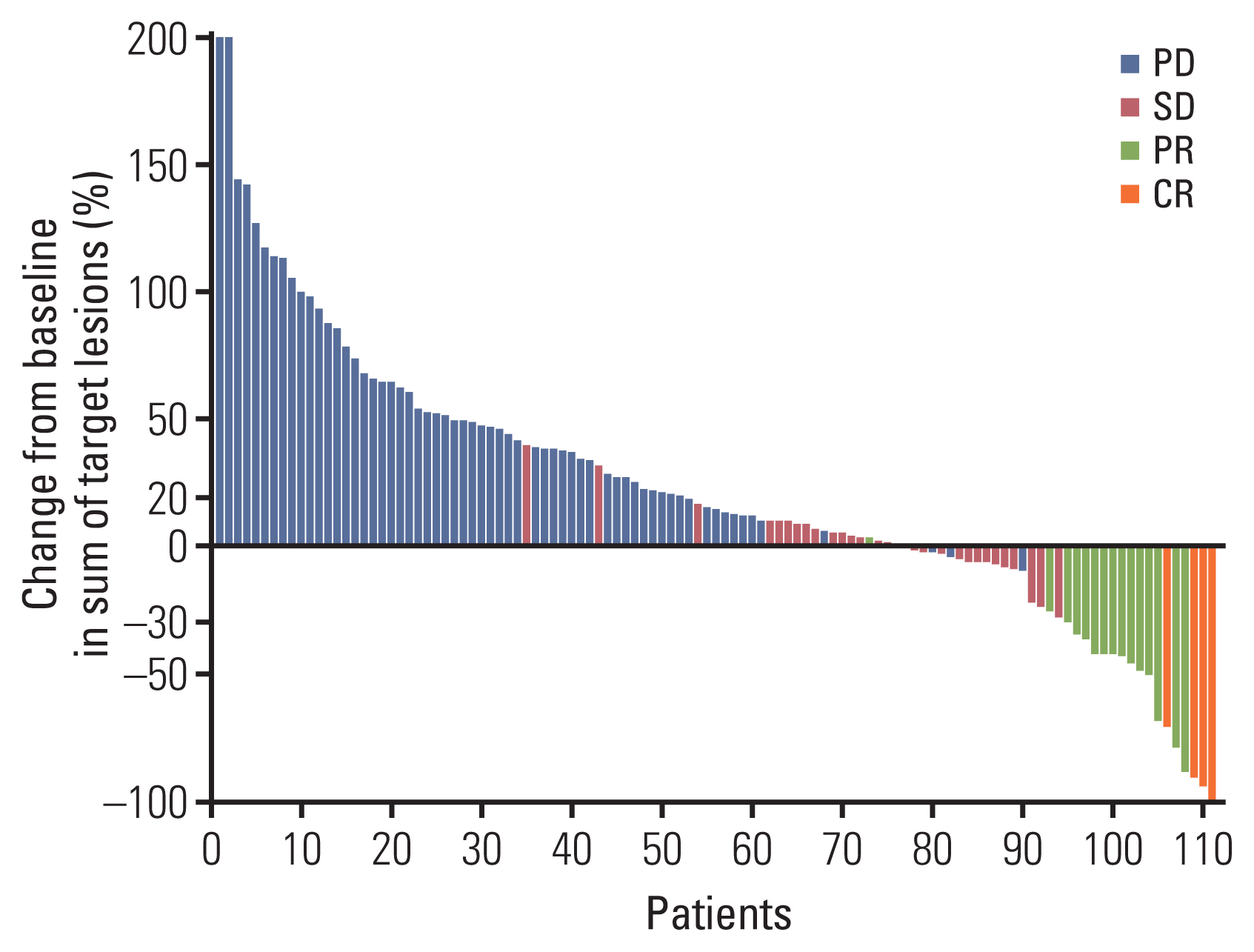
Fig. 2Progression-free survival and overall survival in 123 evaluable patients. (A) Median progression-free survival: 2.7 months (95% confidence interval [CI], 2.2 to 3.5). (B) Median overall survival: 10.8 months (95% CI, 7.5 to 18.7). (C) Overall survival by primary tumor location. (D) Overall survival by oropharynx vs. non-oropharynx (hazards ratio, 0.46; 95% CI, 0.24 to 0.86; p=0.016). 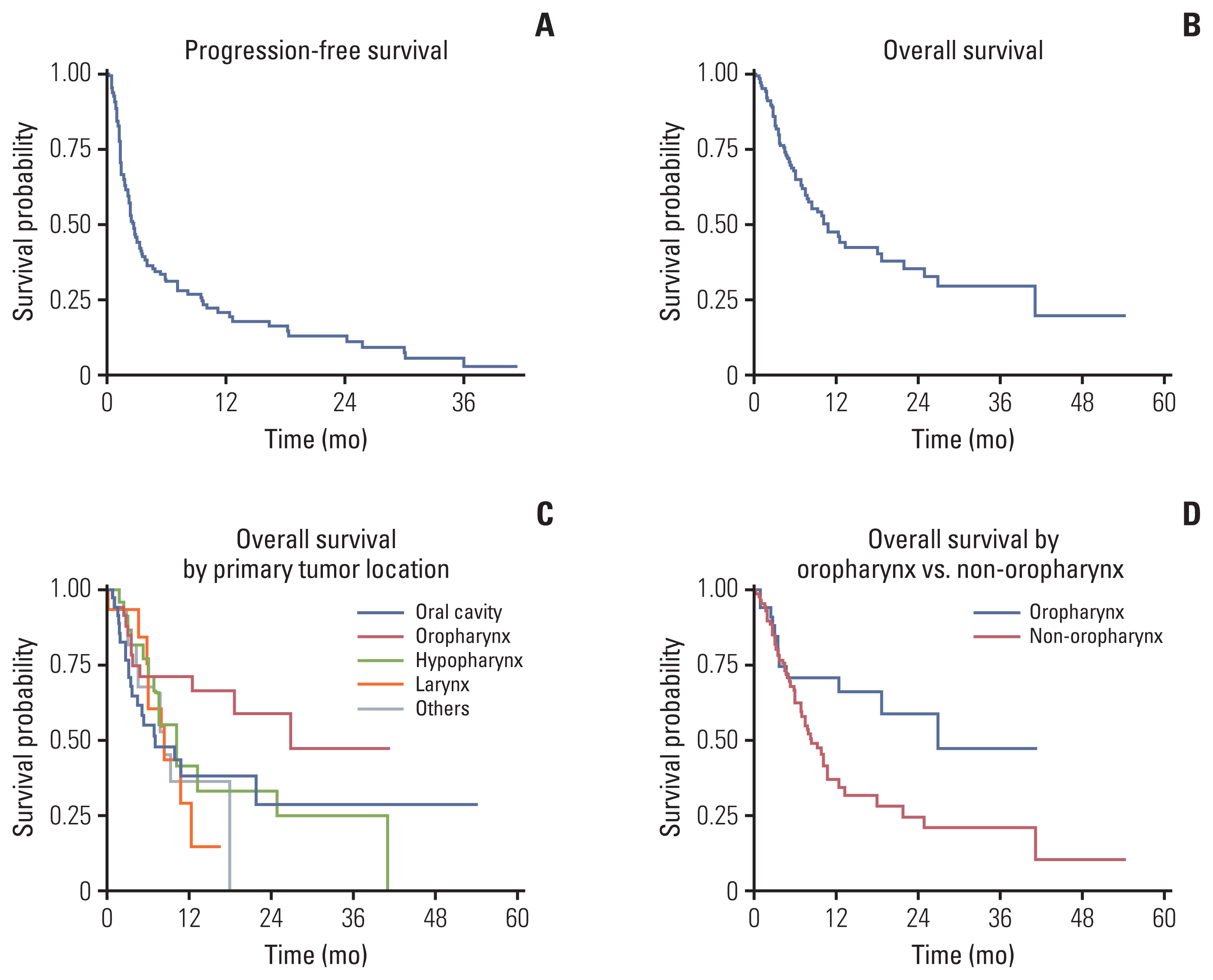
Table 1Baseline demographics and disease characteristics of patients with advanced HNSCC treated with immune checkpoint inhibitors Table 2Characteristics of ICIs which was administered to 125 patients with recurrent and metastatic HNSCC
Values are presented as number (%) or median (range) unless otherwise indicated. CI, confidence interval; CTLA-4, cytotoxic T-lymphocyte antigen 4; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1. Table 3Antitumor activity of ICIs in 125 patients with recurrent and metastatic HNSCC
Table 4Patients and tumor characteristics related to progression-free survival and overall survival according to multivariate analysis
References1. Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw. 2018;16:479–90.
2. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27.
3. Leon X, Hitt R, Constenla M, Rocca A, Stupp R, Kovacs AF, et al. A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol (R Coll Radiol). 2005;17:418–24.
4. Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, Soulieres D, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol. 2009;27:1864–71.
5. Machiels JP, Haddad RI, Fayette J, Licitra LF, Tahara M, Vermorken JB, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–94.
6. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
7. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
8. Cohen EE, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67.
9. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65.
10. Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60.
11. Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–32.
12. Fancello L, Gandini S, Pelicci PG, Mazzarella L. Tumor mutational burden quantification from targeted gene panels: major advancements and challenges. J Immunother Cancer. 2019;7:183.
13. Prat A, Navarro A, Pare L, Reguart N, Galvan P, Pascual T, et al. Immune-related gene expression profiling after PD-1 blockade in non-small cell lung carcinoma, head and neck squamous cell carcinoma, and melanoma. Cancer Res. 2017;77:3540–50.
14. Saloura V, Cohen EE, Licitra L, Billan S, Dinis J, Lisby S, et al. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2014;73:1227–39.
15. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–9.
16. Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24:4960–7.
17. Katsurada M, Nagano T, Tachihara M, Kiriu T, Furukawa K, Koyama K, et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res. 2019;39:815–25.
18. Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7.
19. Cao J, Zhu X, Zhao X, Li XF, Xu R. Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis in prostate cancer: a systematic review and meta-analysis. PLoS One. 2016;11:e0158770.
20. Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology. 2016;16:1080–4.
21. Faria SS, Fernandes PC Jr, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. E cancer medical science. 2016;10:702.
22. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–65.
23. Finotello F, Trajanoski Z. New strategies for cancer immunotherapy: targeting regulatory T cells. Genome Med. 2017;9:10.
24. Zilio S, Serafini P. Neutrophils and granulocytic MDSC: the janus god of cancer immunotherapy. Vaccines (Basel). 2016;4:31.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||