AbstractPurposeThis study investigated the incidence and relative survival rates (RSRs) for cancers among adolescents and young adults (AYAs) aged 15–39 years between 1993 and 2016 in Korea.
Materials and MethodsData from the Korea Central Cancer Registry were used to calculate percent distributions, age-specific incidence rates, age-standardized incidence rates (ASRs) per million, annual percent changes (APCs), average APCs, and RSRs for cancers diagnosed in AYAs.
ResultsASR of all cancers among AYAs was 654.5 per million. The largest diagnosed group of cancers was carcinomas (almost 80%). Crude incidence increased with age, from 170.4 per million for those aged 15–19 years to 1,639.8 per million for those aged 35–39 years. ASR increased from 414.8 per million to 820.4 per million, with an APC of 9.0%. The incidence of thyroid carcinoma showed the most rapid increment (APC, 14.0%), followed by non-Hodgkin lymphoma (APC, 13.4%). The 5-year RSR among AYAs significantly improved from 62.1% to 90.8%. Survival improvement in AYAs was higher than that in children but lower than that in older adults (APC, 2.1% vs. 1.9% vs. 3.1%). The most marked survival improvement was found for leukemia and lymphoma. Astrocytoma, rhabdomyosarcoma, and carcinoma of the trachea, bronchus, and lung had a 5-year RSR of < 50%.
IntroductionCancers in adolescent and young adult (AYA) patients have distinctive characteristics compared to cancers in children and older adults. While studies variably define the AYA population, developmental theories can provide a rationale for assigning an age range. The late teen years to mid-20s represent a period of “emerging adulthood” with the transition from adolescence to adulthood occurring by age 20 [1]. In some instances, age 30 to 39 years serves as an upper boundary for young adult. Recently, the US National Cancer Institute (NCI) proposed the definition of AYAs aged 15–39 years, based on the Surveillance, Epidemiology, and End Results (SEER) data showing that people in this age range have not shared in the improvements in cancer mortality and survival that have been experienced by other patients [2]. A recent authoritative review of cancer epidemiology adopts the age limits of 15–39 years [3].
Studies for AYAs with cancer have revealed that survival among these patients is often lower than that among younger or older patients [4,5]. Of note, there has been less improvement in survival among AYA patients in recent decades [6]. EUROCARE has shown that AYAs aged between 15 and 24 years have poorer survival than children aged under 14 years for most cancers [7]. However, Keegan et al. [8] recently reported cancer survival trends of AYAs between 2002 and 2006 in the United States using SEER database and found that the 5-year relative survival rate (RSR) for all invasive cancers in AYAs aged 15–39 years is 82.5%. In that study, survival in several cancer types occurring in AYAs showed improvement. However, survival did not appear to be improving to the same extent in AYAs as in children for several cancers such as breast cancer and colorectal cancer.
More than a million new diagnoses of cancer are made annually among a global population of 3 billion AYAs worldwide [9]. In Korea, over 200,000 new cancer patients are diagnosed annually, and approximately 15,000 (7.5%) of which are in AYAs [10]. Cancer is the most common cause of death among AYAs in Korea between 1995 and 2018 (http://kostat.go.kr; accessed November 20, 2019). Because only a small proportion of all malignancies are diagnosed in AYAs, AYAs with cancer are neglected in many ways by both pediatric and adult oncologists, yet effective disease management necessitates a multi-professional approach incorporating expertise from multiple specialties [11].
Previously, Moon et al. [12] reported the 5-year survival rate of AYAs with cancer in Korea between 2006 and 2010 was 89.1%. In their study, AYAs were defined as individuals aged 15–29 years. The present study is an update until 2016 using the latest definition of AYAs, which defines them as individuals aged 15–39 years, which facilitates international comparisons. We also tried to demonstrate the shift in the distribution of cancer types in AYAs and compare cancer survival improvement in AYAs with that in children and older adults.
Materials and Methods1. Data collectionWe used data provided by the Korea National Cancer Incidence database in the Korea Central Cancer Registry (KCCR). In 1980, the Korean Ministry of Health and Welfare started the KCCR, a nationwide, hospital-based cancer registry (http://www.ncc.re.kr). Until 1998, the registry annually collected cancer cases from more than 180 hospitals in Korea, these data represent 80%–90% of all cancer incidence in Korea. Since 1999, the KCCR has covered the entire population under the population-based cancer registry program. Cancer sites were coded by primary site and morphology using the International Classification of Diseases for Oncology (3rd edition) [13]. The KCCR contains data on variables including age, sex, first diagnosis date, primary tumor site, morphology, method of diagnosis, and stage at diagnosis. The cancers were registered only if confirmed by physicians, irrespective of whether the tumors were examined microscopically.
Incidence data were collected for Korean AYAs aged 15–39 years who were newly diagnosed with cancer between 1999 and 2016. To estimate cancer survival, data for individuals who were newly diagnosed with cancer between in 1993–2016 were obtained and the survival status of each patient was followed until December 31, 2017. To compare cancer survival trends between AYAs and children and older adults, individuals aged < 15 years and ≥ 40 years were classified as children and older adults, respectively.
2. Case classificationCancers were classified according to the SEER AYA scheme based on a modified version of the International Classification of Childhood Cancer [14]. In particular, the SEER AYA scheme was based on an updated classification of cancers occurring in AYAs proposed by Barr et al. [15]. It is composed of 10 major groups and second- and third-level subgroups according to the site of origin.
3. Statistical methods1) IncidenceAge-specific incidence rates and age-standardized incidence rates (ASRs) using the Segi’s world standard population defined by the World Health Organization were calculated according to sex and age group at diagnosis and expressed per million. Trends in annual ASRs were examined using annual percent change (APC) which was estimated using the following formula: 100×(eβ-1), where β was the slope calculated from a linear regression of log age-standardized incidence rates for the period from 1999 to 2016 [16]. Male-to-female ratio (M/F) was calculated as the ratio of the ASR in males to that in females.
2) SurvivalRSRs were analyzed according to periods of diagnosis and diagnostic group. RSRs were calculated by dividing observed survival by expected survival of the general population which was derived from the standard life table provided by Statistics Korea (http://kosis.kr) using the Ederer II method. These survival rates were estimated using a complete approach that could reflect recent data. Due to this inclusion of early survival experience of more recently recruited patients, the analysis provided more up-to-date and precise long-term survival rates [17]. APC and average APC (AAPC) of 5-year RSR were calculated. AAPC, as a summary measure of the trend over a pre-specified fixed interval, was computed as a weighted average of APCs, with weights equal to the length of the APC interval [18]. Analyses of incidence and survival were performed using SAS ver. 9.4 (SAS Institute, Inc., Cary, NC). Analyses of trends were conducted using Joinpoint 4.7.0.0 (National Cancer Institute, Bethesda, MD).
Results1. IncidenceBetween 1999 and 2016, there were 270,640 new cases of cancer among AYAs, with a crude incidence of 779.6 per million and an ASR of 654.5 per million. The crude incidence increased with age: 170.4 per million at 15–19 years of age, 275.1 at 20–24 years, 557.4 at 25–29 years, 1,030.6 at 30–34 years, and 1,639.8 at 35–39 years (Table 1). A female predominance was found with a male to female ratio of 0.4 (Fig. 1). This predominance was largely due to a much higher incidence of carcinomas of the thyroid, breast, and genitourinary tract (M/F, 0.2, 0.0, and 0.2, respectively).
Even among AYAs, the spectrum of cancers observed differed according to age (Table 1). In patients aged 15–19 years, carcinoma, leukemia, lymphoma, and central nervous system (CNS) tumor accounted for 30%, 17%, 12%, and 8% of all cancers, respectively. With increasing age, the incidence of carcinomas increased while incidences of leukemia, lymphoma, and CNS tumors were decreased. The percentage of carcinomas among all cancers was 57% in patients aged 20–24 years, 75% in patients aged 25–29 years, 84% in patients aged 30–34 years, and 87% in patients aged 35–39 years.
Carcinoma, unspecified malignant neoplasms, and miscellaneous specified neoplasms occurred more often in females, with M/F of 0.3, 0.7, and 0.8, respectively. On the contrary, other cancer categories including leukemia, lymphoma, CNS neoplasms, osseous and chondromatous neoplasms, and germ cell and trophoblastic neoplasms showed male predominance with M/F of 1.3, 1.2, 1.2, 1.5, and 1.5, respectively (Table 1).
Between 1999 and 2016, the largest diagnosed group of cancers consisted of carcinoma (group 8) representing almost 80% of all cancers, followed by lymphoma and leukemia (4% each). Among carcinomas, thyroid carcinoma was the most common carcinoma, with approximately 102,600 diagnosed and a crude incidence of 295.7 per million accounting for 47% of all carcinomas and 38% of all cancers, followed by carcinoma of gastrointestinal tract (21.0%) and breast carcinoma (12.0%) of all cancers. Notably, thyroid carcinoma was approximately five times more common in females than in males, with an ASR of 413.2 per million in females and 89.0 per million in males. Because the incidence of thyroid carcinoma was unusually high, the ASR of all cancers combined was recalculated, excluding thyroid carcinoma. Without thyroid carcinoma, the ASR of all other cancers was 407.4 per million (314.3 per million in males and 504.4 per million in females) (Table 1).
Fig. 2A shows trends of cancer incidence among AYAs from 1999 to 2016. The APC during that time period was 9.0% (p < 0.05, 8.2% in males and 9.2% in female). Although the ASR increased from 414.8 per million in 1999 to 820.4 per million in 2016, it temporarily declined in 2014 and 2015. This decline was due to a decrease in newly diagnosed thyroid cancers during this period. Excluding thyroid carcinomas, the APC in cancers among AYAs was 5.7% (5.4% in males and 5.8% in females) without temporary decline in 2014 and 2015 (Fig. 2B).
The incidence of most cancer types showed statistically significant increasing trend except for CNS neoplasms (group 3) and miscellaneous specified neoplasms (group 9) (S1–S3 Tables). Cancers with annual increases over 10% during the study period were thyroid carcinoma in males and females (APC, 14.1% and 12.9%; p < 0.05, respectively), acute lymphoid leukemia (ALL) in males (APC, 12.9%; p < 0.05), non-Hodgkin lymphoma (NHL) in males and females (APC, 11.4% and 13.9%; p < 0.05, respectively), germ cell and trophoblastic neoplasm of gonads in males (APC, 10.7%; p < 0.05) and carcinoma of colon and rectum in males (APC, 10.4%; p < 0.05). By contrast, the incidence of all CNS neoplasms (group 3) showed no significant change over the study period.
2. SurvivalWe estimated 5-year RSR according to the following periods: 1993–1995, 1996–2000, 2001–2005, 2006–2010, and 2012–2016 (Fig. 3). The 5-year RSR among AYAs markedly improved from 62.1% in 1993–1995 to 90.8% in 2012–2016 (p < 0.05) (S4 Table). By sex, the degree of survival improvement was 37.3% (p < 0.05) in males and 22.7% (p < 0.05) in females. The survival rate was slightly lower in males than that in females over the time period (S5 and S6 Tables).
All 10 diagnostic groups of AYA cancers showed statistically significant improvements in 5-year survival over the time period (Fig. 4). The most marked improvements were found for leukemia (group 1) and lymphoma (group 2): from 25.8% and 56.9% in 1993–1995 to 68.4% and 88.7% in 2012–2016 (p < 0.05, each), respectively (S4 Table). Among leukemias, chronic myelogenous leukemia (CML) showed the greatest increase in survival from 1993–1995 to 2012–2016 in both males (39.1% to 96.1%, p < 0.05) and females (38.0% to 97.1%, p < 0.05). Survival for CNS neoplasms (group 3) improved from 54.2% in 1993–1995 to 65.5% in 2012–2016 (p < 0.05). Among CNS neoplasms, low-grade astrocytoma (group 3.1.1), glioma and anaplastic astrocytoma (group 3.1.2), ependymoma (group 3.3), and medulloblastoma and primitive neuroectodermal tumor (PNET) (group 3.4) showed no survival improvement over the study period. Survival rates for thyroid carcinoma (group 8.1) and skin carcinoma (group 7.2) were very high across all time periods (from 99.5% and 81.9% in 1993–1995 to 100% and 94.7% in 2012–2016, respectively). When survival was considered for all cancers except thyroid cancer, AYAs had 5-year RSRs of 58.4% (46.0% in males and 66.5% in females) in 1993–1995 and 81.4% (76.1% in males and 84.5% in females) in 2012–2016, with a change of 23.0% over the study period (p < 0.05).
As of 2012–2016, cancers with > 90% 5-year survival rates were thyroid carcinoma (group 8.1, 100%), germ cell and trophoblastic neoplasms of gonads (group 6.2, 97.3%), CML (group 1.3, 96.4%), Hodgkin lymphoma (group 2.2, 95.4%), skin carcinoma (group 7.2, 94.7%), carcinoma of breast (group 8.4, 91.4%), and other specified and unspecified bone tumors (group 4.4, 91.1%). By contrast, the following cancers had survival rates < 60%: astrocytoma (group 3.1, 46.0%), rhabdomyosarcoma (group 5.2, 47.0%), carcinoma of trachea, bronchus, and lung (group 8.3, 48.6%), ALL (group 1.1, 52.6%), and Ewing tumor (group 4.3, 56.3%) (S4 Table).
Fig. 5 shows the 5-year survival trends in children, AYAs, and older adults, with Joinpoint regression analysis to identify inflection years and quantitate survival trends. For all cancers combined, survival improvement in AYAs was higher than that in children but lower than that in older adults (AAPC, 2.1 vs. 1.9 vs. 3.1), with survival rates being the highest in 2012 (91.3% in AYAs, 82.5% in children, and 69.5% in older adults) (Fig. 5A). Because the spectrum of cancers observed in AYAs differed according to age, we subdivided the AYA group into 15–19 years old, 20–29 years old, and 30–39 years old groups (Fig. 5B). Of these groups, the degree of survival improvement was the highest in the 15–19 years old group but the lowest in the 30–39 years old group (AAPC, 2.5 vs. 2.1). Survival was the highest in both the 20–29 years old and 30–39 years old groups as of 2012. The survival trend curve of the 15–19 years old group was very similar to that in children, while the survival trend curve of the 20–29 years old group was very similar to that of the 30–39 years old group. Excluding thyroid cancer, survival improvement was the highest in older adults, followed by that in AYAs and children (AAPC, 2.7 vs. 1.9 vs. 1.7) (Fig. 5C). Among AYA groups, the survival improvement was the highest in patients aged 15–19 years (AAPC, 2.8), followed by patients aged 20–29 years (AAPC, 2.3) (Fig. 5D). Patients aged 15–19 years showed an overall worse prognosis than did those aged 20–39 years, but they showed the highest degree of survival improvement regardless of the inclusion of thyroid cancer. As of 2012, the 5-year survival rate was 81.8% in children, 80.7% in AYAs (77.6% in those aged 15–19 years, 81.5% in those aged 20–29 years, and 80.8% in those aged 30–39 years), and 63.0% in older adults (Fig. 5D).
DiscussionIn this study, we provided up-to-date data on cancer incidence, survival, and trends among AYAs in Korea. Our study revealed that the ASR of cancers in AYAs was 654.5 per million, with an APC of 9.0% between 1999 and 2016. Cancer incidence was approximately 10 times greater in patients aged 35–39 years compared to that in those aged 15–19 years. In 2016, the ASR of cancers in AYAs was 820.4 per million in Korea, which was higher than that in France (ASR, 751), the United Kingdom (ASR, 658), the United States (ASR, 640), or Japan (ASR, 492) (https://gco.iarc.fr/today; accessed December 4, 2019).
The spectrum of cancers in AYAs is distinct from that in younger and older populations [19]. Among AYAs, the spectrum of cancers differs according to age [8,20]. Concordantly, we also found a shift in the distribution with increasing age within the AYA group, with the expansion of the AYAs to those aged 15 to 39 years. With an increase in age, proportions of those with leukemias, lymphomas, and CNS tumors decreased whereas the proportion of carcinomas increased.
The higher incidence of cancers in Korean AYAs was mainly due to a high incidence of thyroid cancer. Of cancers in AYAs, the incidence of thyroid carcinoma had the greatest increase, nearly 10-fold in 2013 (ASR, 504.1) compared to that in 1999 (ASR, 47.7). There was a greater increase in thyroid cancer among female AYAs than in male AYAs. An increase in the incidence of thyroid carcinoma has also been noted among AYAs in Western countries [9]. However, the incidence of thyroid carcinoma among AYAs is much higher in Korea than that in other countries [21–23]. According to SEER13 data, thyroid carcinoma was diagnosed in 12.1% of cancers in AYAs [8]. In the Middle East, thyroid carcinoma was diagnosed only in 9.0% of Arabic adolescents and 7.9% of Jewish adolescents during 1998–2009 [24]. The high incidence of thyroid carcinoma might be linked to ethnical and environmental factors unique to Korea. However, the increase in thyroid cancer incidence seems to be primarily due to overdiagnosis, meaning the diagnosis of this disease that looks like is labeled as cancer, although it will not affect the person in his/her lifetime as shown in this study. Since thyroid cancer has nearly a 100% survival rate, the overall cancer survival rate is inflated. Overdiagnosis of thyroid cancer is attributed in part to the increasing availability and application of more sensitive diagnostic instruments [25]. The Korean medical system and health-care policy that encourage cancer screening could be one reason for the increase in the incidence of thyroid carcinoma. As a result of effort to reduce overdiagnosis of thyroid carcinoma, the incidence of cancers diagnosed in AYA declined in 2014 and 2015, which was due to a temporary decrease in newly diagnosed thyroid cancers during this period. After excluding thyroid carcinoma, the APC of cancer incidence in AYAs decreased to 5.7%. The ASR in 2016 was 407.4 per million.
NHL in females showed the second greatest increase in incidence, with an APC of 13.9%, followed by ALL in males and NHL in males. The reason for a sharp increase in hematologic malignancies such as ALL and NHL in Korean AYAs remains unknown. Bleyer et al. [9] reported that the increasing incidence of NHL and ALL in AYAs might be due to a sexually transmitted human papillomavirus (HPV). However, HPV associated lymphoma is rare in Korea. Furthermore, HPV vaccines for females have been recommended since 2007 and the incidence of cervical carcinoma has not significantly increased between 1999 and 2016, indirectly suggesting that the prevalence of HPV infection has not increased. Therefore, further research is needed to identify associated risk factors including environmental exposure to determine the reason for the increasing incidence of NHL and ALL in Korean AYAs.
When looking at incidence for all cancers combined in AYAs, a female predominance was found, with sex-specific disequilibrium increasing with age. Thyroid, breast, and cervix cancers, the three most common cancers, affected exclusively or mostly females. This phenomenon was also found worldwide [9]. Previously, Cook et al. [26] reported higher cancer mortality in males than in females, and they suggested that sex-related cancer disparities are more strongly related to etiology than prognosis. In our study, although female AYAs had higher incidences, males had lower survival rates. This phenomenon is mainly due to a higher prevalence of cancers with good prognoses in females than in male. Survival rates of thyroid carcinoma and breast carcinoma as the two most common cancers in females were > 90%. Overdiagnosis through screening of these tumors might also have an effect on cancer survival.
The sex disparity in thyroid cancer incidence is well-established [27,28]. The fluctuation of sex hormones during a woman’s menstrual cycle and pregnancy has been hypothesized as the reason for the sex disparity in thyroid cancer. Upon the onset of puberty, the incidence of thyroid cancer increases in females only and declines again after menopause. Recent studies demonstrated that estradiol, the main female sex hormone, is a potent stimulator of both benign and malignant human thyroid cells. Additionally, polymorphisms in the estrogen receptor could be a risk factor for thyroid cancer [29]. Similarly, Rajoria et al. [30] documented that estrogen was associated with increased adherence, invasion, and migration in thyroid cancer cell lines, which suggests that the estrogen receptor status is affected by sex hormones. No established molecular factors appear to explain sex differences in thyroid cancer.
In Korea, the 5-year RSR for 2012–2016 was 90.8% in AYAs for all cancers and 81.4% in AYAs for cancers excluding thyroid carcinoma. Such level of survival is not inferior to the survival rate for childhood cancers in Korea [31]. It is similar to those reported for AYAs in Europe and the United States (approximately 82%) [8,20].
In the current study, it is encouraging to observe progress in survival rates for some cancers among AYAs, particularly CML and acute myeloid leukemia (AML) and, to a lesser extent, NHL, breast cancer, and colorectal cancer. The substantial improvement in survival for CML and AML is likely due to major advances in therapy, including targeted therapy with tyrosine kinase inhibitors for CML. Similarly, the improvement in NHL survival rate is likely due to the development of monoclonal antibody therapy. However, survival rates among AYAs were lower for several cancers relatively common in children such as ALL, rhabdomyosarcoma and Ewing tumor. For cancers in AYAs that also affect children, it has been suggested that AYAs should be treated in an integrated pediatric-adult multidisciplinary setting [11]. This change should increase the likelihood of AYAs being included in clinical trials, and improve family and social support. For ALL, application of treatment protocols for children is feasible. It can improve outcomes. Our results indicated that the survival rate for ALL in AYAs was improved by 34.3% between 1993 and 2016. However, the 5-year RSR for ALL in AYAs was 52.6% in 2012–2016, whereas the 5-year RSR for ALL in children was above 80% [31]. This might indicate that the biology of the host plays a role in determining the outcome of treatment for cancers in AYAs although progress has been made in ALL treatment. The survival rate (47.0%) for rhabdomysarcoma in AYAs is also disappointing. Rhabdomyosarcoma-specific survival was 78.8% among children in Korea [31]. Biologic research and clinical trials focusing on AYAs should be a priority for future research. Additionally, hormonal and pharmacological considerations are worthy of attention for this age group.
Breast cancer under the age of 40 has been frequently associated with inferior outcomes due to the greater risk of developing triple-negative (i.e., estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 negative) with stromal related signature, and pathologic grade 3 tumors, as well as vascular or lymphatic invasion [32,33]. However, due to more aggressive adjuvant treatments including radiation, chemotherapy, and biological and endocrine therapy, the survival rate of AYAs with breast carcinoma is improving. The retrospective evaluation of AYAs with cancer in the SEER 13 database found that the survival rate of AYAs with breast carcinoma was 85.5% which was reported previously to have worse outcomes in AYAs than older adults [8]. Similarly, we found that the 5-year RSR of breast carcinoma increased from 79.4% in 1993–1995 to 91.4% in 2012–2016. Recently, Johnson et al. [33] reported that Asian AYAs with breast carcinoma had the lowest mortality rates compared to African Americans, white non-Hispanic, Hispanic, and Native North Americans. This implies that an ethnic factor could be involved in the high survival rate in AYAs with breast cancer in our study.
CNS neoplasms (group 3) comprised only 2% of all cancers in AYAs. They showed no significant survival change over the study period. Survival rates for subgroups of CNS tumors were similar to those reported in the United States and Germany [8,34]. In our study, low-grade astrocytoma, glioma and anaplastic astrocytoma, ependymoma, medulloblastoma and PNET showed no survival improvement over the study period. Furthermore, glioblastoma, anaplastic astrocytoma, and PNET showed the worst survival rate in AYAs with cancer. Currently, there have been no reports comparing the incidence or survival rates for CNS tumors between children and older adults using disease categories used in the present study. Considering that these cancers had worse outcomes than other cancers in AYAs, progress needs to be made through a combination of methods that lead to improved clinical outcomes by advancing risk stratification and treatment protocols through clinical trials of AYAs with CNS tumors.
We found that cancers in AYAs demonstrated survival improvement comparable to that achieved in children. Additionally, survival rates for AYAs were higher than those for older adults. These results suggest that survival deficit among AYA patients does not exist in Korea. Although patients aged 15–19 years have an overall worse prognosis than 20–39-year-olds, they were catching up based on the Joinpoint model with highest degree of survival improvement. Why the survival in those aged 15–19 years is lower than that in those aged 20–39 years should be investigated. Common cancers occurring among 15–19-year-olds are different from those occurring in adulthood, but similar to those occurring in children. The survival improvement in 15–19-year-olds is due, at least in part, to the increased awareness of the worse survival, and the delivery of better therapies to this group [35,36].
In conclusion, our study provides representative cancer statistics in Korean AYAs. Results of this study showed an increasing trend in cancer incidence and differences in cancer distribution according to age and sex. In particular, research on the etiology of the high prevalence of thyroid carcinomas in AYAs is required. We could not find a survival disadvantage for AYA patients compared to other age groups. However, survival is still lagging in some tumors such as CNS neoplasms, ALL, and Ewing tumors. More vigorous studies on risk factors affecting cancer incidence and responses to treatment among AYAs are needed to improve cancer outcomes. Furthermore, investment in exploring the distinct biology of tumors in AYAs must be a priority in AYA oncology.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board (IRB) of the National Cancer Center (IRB No. NCC 2020-0079). Informed consent was waived because of the retrospective nature of the study using database in the Korea Central Cancer Registry. AcknowledgmentsThis work was supported by a research grant (No.1910132) from the National Cancer Center, Republic of Korea. Special thanks to the tumor registrars (health information managers) of the Korea Central Cancer Registry (KCCR)-affiliated and non-KCCR-affiliated hospitals for data collection, abstracting, and coding.
Fig. 2Trends of age-standardized incidence rates from 1999 to 2016: all cancers combined (A) and cancers excluding thyroid carcinoma (B). APC, annual percent change. 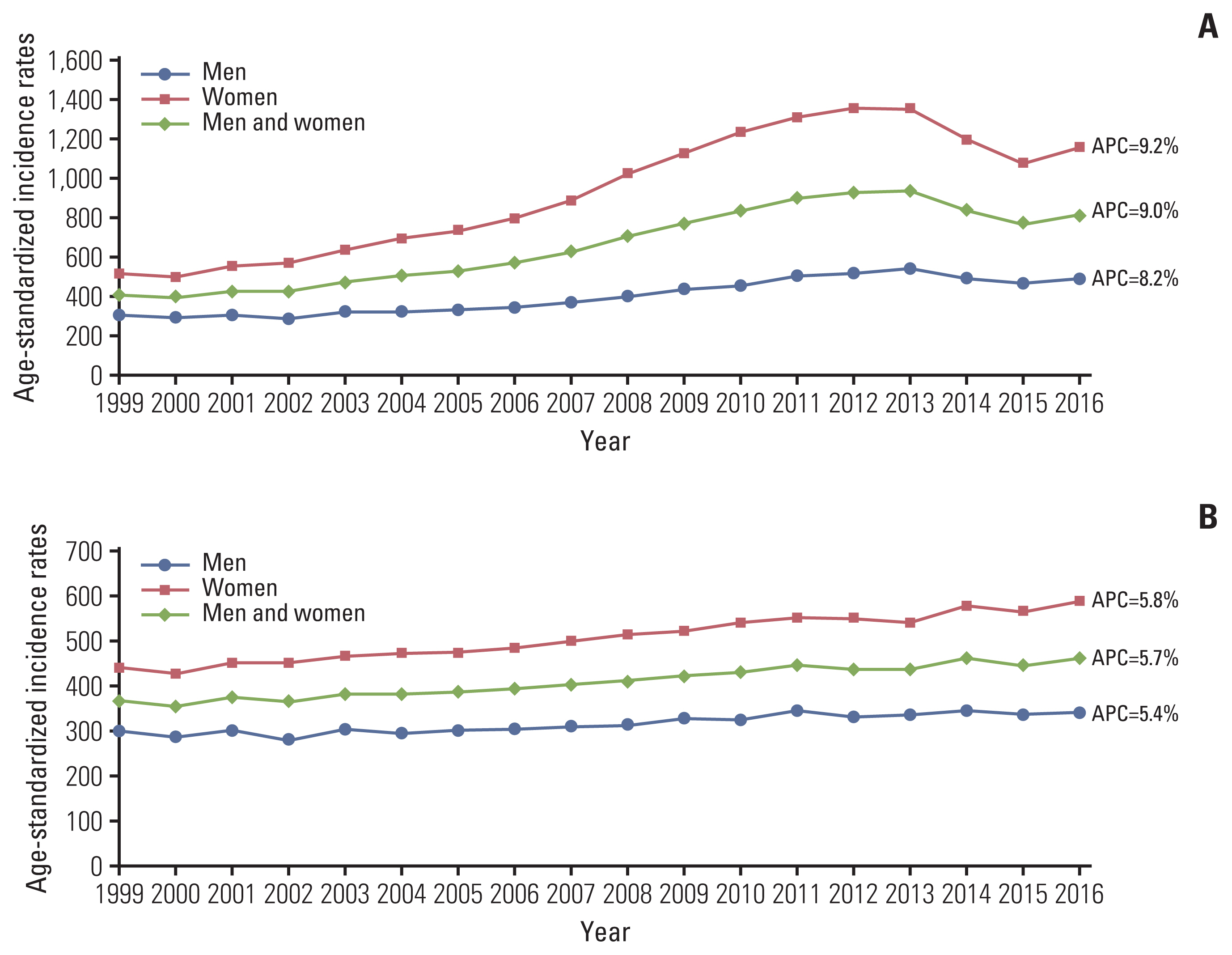
Fig. 4Trends of 5-year survival rate according to diagnostic group in adolescents and young adults. CNS, central nervous system. 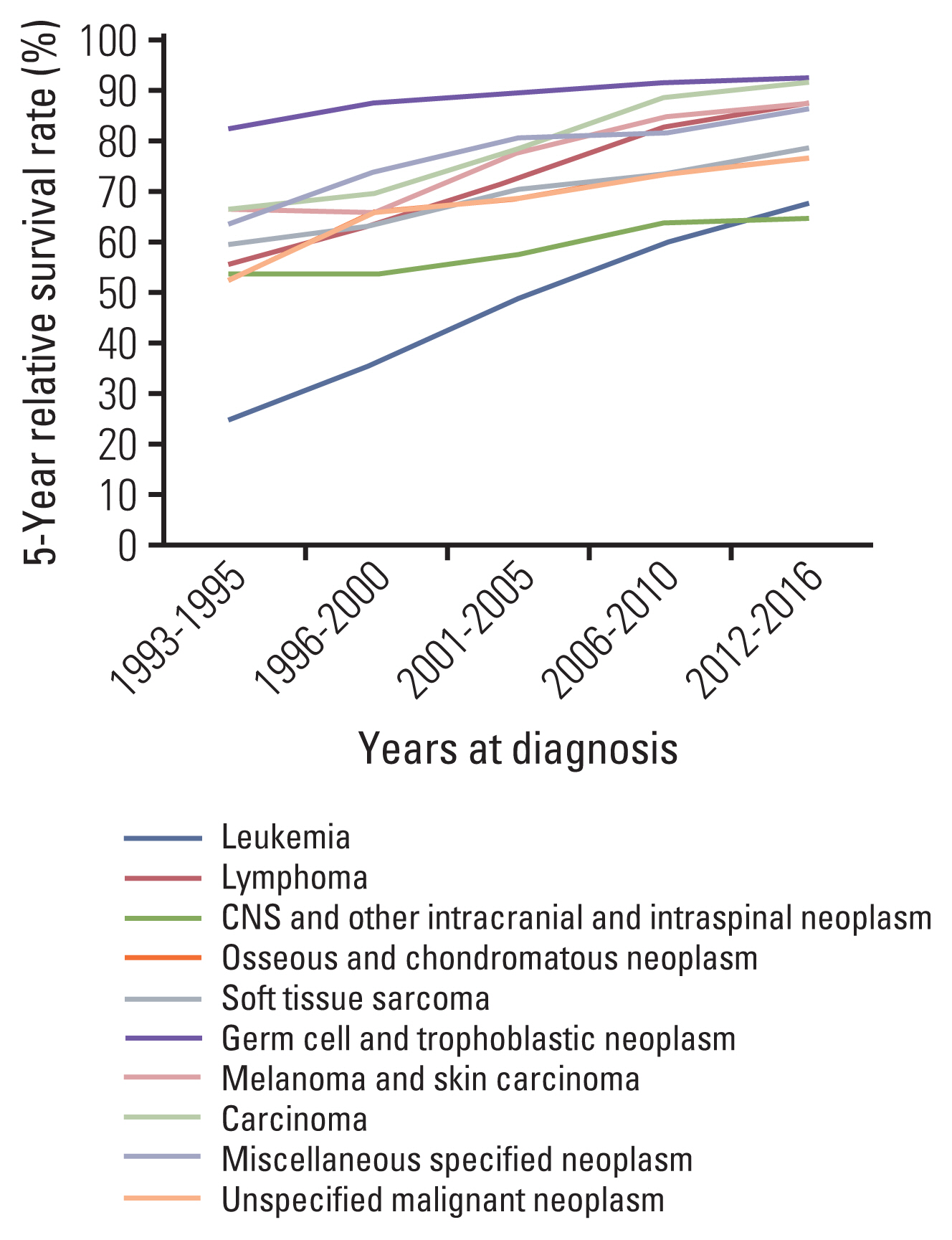
Fig. 5Annual 5-year survival trends in patients aged < 15 years, 15–39 years, and ≥ 40 years for all cancers combined (A), patients aged < 15 years, 15–19 years, 20–29 years, 30–39 years, and ≥ 40 years for all cancers combined (B), patients aged < 15 years, 15–39 years, and ≥ 40 years in cancers excluding thyroid carcinoma (C), and patients aged < 15 years, 15–19 years, 20–29 years, 30–39 years, and ≥ 40 years in cancers excluding thyroid carcinoma (D). AAPC, average annual percent change; APC, annual percent change. 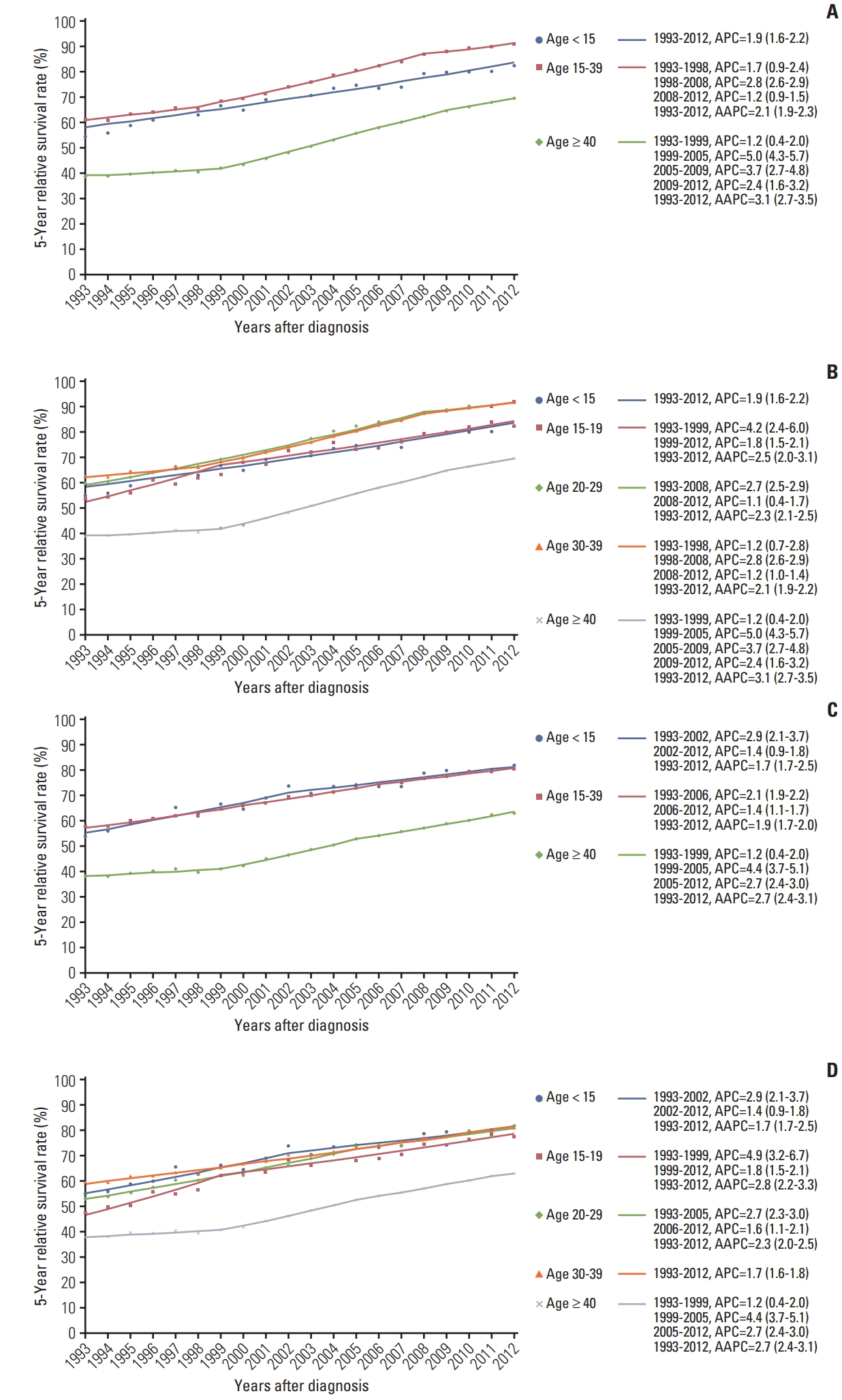
Table 1Cancer incidences per million by age group in adolescents and young adults
References1. Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55:469–80.
2. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute and the LiveStrong Young Adult Alliance. Adolescent and Young Adult Oncology Progress Review Group: closing the gap: research and care imperatives for adolescents and young adults with cancer. NIH Pub No. 06-6067. Bethesda, MD: National Cancer Institute; 2006.
3. Desandes E, Stark DP. Epidemiology of adolescents and young adults with cancer in Europe. Prog Tumor Res. 2016;43:1–15.
4. Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57:242–55.
5. Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110:2385–93.
6. Bleyer A, Choi M, Fuller CD, Thomas CR Jr, Wang SJ. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol. 2009;36:460–7.
7. Gatta G, Zigon G, Capocaccia R, Coebergh JW, Desandes E, Kaatsch P, et al. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur J Cancer. 2009;45:992–1005.
8. Keegan TH, Ries LA, Barr RD, Geiger AM, Dahlke DV, Pollock BH, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–16.
9. Bleyer A, Ferrari A, Whelan J, Barr RD. Global assessment of cancer incidence and survival in adolescents and young adults. Pediatr Blood Cancer. 2017;64:e26497.
10. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14.
11. Stark D, Bielack S, Brugieres L, Dirksen U, Duarte X, Dunn S, et al. Teenagers and young adults with cancer in Europe: from national programmes to a European integrated coordinated project. Eur J Cancer Care (Engl). 2016;25:419–27.
12. Moon EK, Park HJ, Oh CM, Jung KW, Shin HY, Park BK, et al. Cancer incidence and survival among adolescents and young adults in Korea. PLoS One. 2014;9:e96088.
13. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000.
14. Bleyer A, O’Leary M, Barr R, Ries LA. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidece and survival: 1975–2000. NIH Pub No. 06-5767. Bethesda, MD: National Cancer Institute; 2006.
15. Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106:1425–30.
16. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2010 [Internet]. Bethesdsda, MD: National Cancer Institute; 2013. [cited 2020 Sep 29]. Available from: https://seer.cancer.gov/archive/csr/1975_2010/
17. Brenner H, Gefeller O. Deriving more up-to-date estimates of long-term patient survival. J Clin Epidemiol. 1997;50:211–6.
18. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51.
19. Barr RD, Ferrari A, Ries L, Whelan J, Bleyer WA. Cancer in adolescents and young adults: a narrative review of the current status and a view of the future. JAMA Pediatr. 2016;170:495–501.
20. Trama A, Botta L, Foschi R, Ferrari A, Stiller C, Desandes E, et al. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: population-based data from EUROCARE-5. Lancet Oncol. 2016;17:896–906.
21. Alston RD, Geraci M, Eden TO, Moran A, Rowan S, Birch JM. Changes in cancer incidence in teenagers and young adults (ages 13 to 24 years) in England 1979–2003. Cancer. 2008;113:2807–15.
22. Marrett LD, Frood J, Nishri D, Ugnat AM; Cancer in Young Adults in Canada Working Group. Cancer incidence in young adults in Canada: preliminary results of a cancer surveillance project. Chronic Dis Can. 2002;23:58–64.
23. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156:167–72.
24. Berkun L, Rabinowicz R, Barchana M, Liphshiz I, Linn S, Futerman B, et al. Cancer incidence and survival among adolescents in Israel during the years 1998 to 2009. Pediatr Blood Cancer. 2013;60:1848–54.
25. Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet. 2014;384:1848.
26. Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–37.
27. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014;21:T273–83.
29. Rebai M, Kallel I, Charfeddine S, Hamza F, Guermazi F, Rebai A. Association of polymorphisms in estrogen and thyroid hormone receptors with thyroid cancer risk. J Recept Signal Transduct Res. 2009;29:113–8.
30. Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41.
31. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016;48:869–82.
32. Tichy JR, Lim E, Anders CK. Breast cancer in adolescents and young adults: a review with a focus on biology. J Natl Compr Canc Netw. 2013;11:1060–9.
33. Johnson RH, Anders CK, Litton JK, Ruddy KJ, Bleyer A. Breast cancer in adolescents and young adults. Pediatr Blood Cancer. 2018;65:e27397.
34. Gondos A, Hiripi E, Holleczek B, Luttmann S, Eberle A, Brenner H, et al. Survival among adolescents and young adults with cancer in Germany and the United States: an international comparison. Int J Cancer. 2013;133:2207–15.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||