AbstractPurposeThis study was undertaken to investigate in detail the xenograft mouse orthotopic lung cancer model induced by PC14PE6 adenocarcinoma cells.
Materials and MethodsThree cell doses (0.5×106; 1×106; 2×106) of PC14PE6 cells were injected into the lungs of male BALB/c nude mice by the intrathoracic injection method. The lung and other organs, including brain, liver, spleen, kidney, muscle, adrenal gland, and lymph node on knee, were removed and stained with H/E to detect the presence of tumor cells.
ResultsThe reliable tumorigenicity time in the PC14PE6 adenocarcinoma cell-inoculated BALB/c nude mouse was 10 days after intrathoracic injection. The average life span of the three groups after inoculation was 14 days in the 2×106 cells inoculum group; 25 days in the 1×106 cells inoculum group; and 32 days in the 0.5×106 cells inoculum group. The PC14PE6 adenocarcinoma cells induced orthotopic lung cancer limited within the thorax.
INTRODUCTIONLung cancer is the major cause of cancer-related deaths worldwide, and its annual mortality exceeds that of breast, prostate, and colorectal cancers (1). Approximately two-thirds of lung cancer patients are initially diagnosed with inoperable disease in which systemic chemotherapy plays an important role in treatment (2). Although there has been progress in systemic chemotherapy with the introduction of novel agents in the past decade, improvement in the survival rate of lung cancer is still needed along with more efficient therapeutic tools. Therefore, a new approach to decreasing the mortality of lung cancer is needed.
One of the most useful tools for investigating and discovering efficacious and novel treatments is the animal cancer model. Animals such as athymic mice have no immune reaction to human cancer cells, which enables researchers to implant cancer cells into the bodies of these animals (3). There are two kinds of xenograft animal cancer models that are frequently used by many researchers: ectopic and orthotopic animal models. An ectopic cancer model is one in which the tumor is implanted subcutaneously, whereas an orthotopic cancer model is one in which cancer tissues are implanted in the analogous or orthotopic sites in the animals (4). As originally depicted by Pagets' "seed and soil" theory, the ectopic cancer model is often criticized for not representing cancer in reality, since the microenvironment plays a crucial role in the development of tumor cells (5). However, because of its availability as a screening tool and the rapidity of tumor development after inoculation, this model has been widely adopted in testing the sensitivities of various drugs (6,7).
However, Onn et al reported that the ectopic lung cancer model might be less susceptible to treatment with paclitaxel compared to the orthotopic lung cancer model, suggesting that orthotopic models are more appropriate for evaluating chemotherapeutics for human lung cancer (8). Furthermore, many human and animal tumors transplanted into nude mice metastasize only if placed in the orthotopic organ, since a subcutaneous environment frequently precludes tumors from metastasizing (9,10).
Therefore, in order to develop an optimal xenograft animal model to assess the efficacy of novel agents, an orthotopic mouse model for non-small cell lung cancer (NSCLC) was established. The properties of the orthotopic lung cancer model in BALB/c nude mice, such as the cancer formation time and the life span of the animals following transplantation, were delineated in this study.
MATERIALS AND METHODS1) Cell lines and tissue culturePC14PE6 cells, the human lung adenocarcinoma cell line, were maintained in MEM (Hyclone) supplemented with 10% heat-inactivated fetal bovine serum and penicillin/streptomycin. Cells were cultured in an incubator with a humidified atmosphere of 5% CO2 in air at 37℃.
2) Animals and animal careMale BALB/c nude mice weighing 20~25 g (Orient, Korea), were maintained at 25℃ with a standard diet and water freely at least 10 days before experiments. Animals were maintained under pathogen-free conditions during experiments. All animals received humane care in compliance with the "Guide for the Care and Use of Laboratory Animals" prepared by the Institute of Laboratory Animal Resources published by the National Institutes of Health and according to the Animal Experiment Guidelines of Samsung Biomedical Research Institute.
3) Marigel and preparation of cell suspension for intrathoracic injectionIntrathoracic injections were performed following previous protocol with minor modifications (8). Briefly, the PC14PE6 tumor cells were harvested from subconfluent culture dishes by mechanical pipetting. The cells were washed once in serum-free medium, resuspended in PBS, and floating cells were collected after centrifugation at 4,000×g for 5 min. Trypan blue staining was used to assess cell viability. Single-cell suspensions of >90% viability were used for injections. The cells were resuspended in HBSS (H4385, Sigma) as a half volume of inoculum and diluted with an equal volume of growth factor-reduced Matrigel (Becton Dickinson & Co., San Jose, CA). Matrigel, a basement membrane matrix preparation, was extracted from Engelbreth-Holm-Swarm mouse sarcoma. Matrigel rapidly generates a gel formation at room temperature or above and acts as an anchor to prevent tumor cells from diffusing and spreading in the thorax after intrathoracic injection. Cell suspensions and injection kit were kept on ice until injection. Three doses of tumor cell inoculums (0.5×106/70 µl; 1×106/70 µl; 2×106/70 µl) were used in the experiment.
4) Intrathoracic injectionMice were anesthetized with an intraperitoneal injection of an aqueous solution (5 ml/kg) of xilazine (8 mg/kg) and ketamine (120 mg/kg) and were placed in the left lateral decubitus position. One-ml tuberculin syringes (Becton Dickinson) with 30-gauge hypodermic needles were used to inject the cell inoculum percutaneously into the right lateral thorax, at the lateral dorsal axillary line, approximately 1.5 cm above the lower rib line just below the inferior border of the scapula. The needle was quickly advanced approximately 6 mm into the thorax and was quickly removed after the injection of 70 µl cell suspension. After tumor injection, the mouse was turned to the right lateral decubitus position. Animals were observed for 30 min until fully recovered.
5) Autopsy, tissue preparation, and H/E stainingMice were sacrificed under anesthesia every 3 days beginning 4 days after inoculation. Following thoracotomy, thoracic organs were removed en bloc, including all of the lymph nodes and tumors. After dissection and removal of the heart, both lungs, including tumor mass, were fixed in formalin and embedded in paraffin. Other organs such as brain, liver, spleen, kidney, muscle, adrenal gland, and lymph node on knee were also removed, fixed in formalin and embedded in paraffin to inspect for the presence of metastases. Whole organs were serially sectioned into 2-mm thick slices and embedded in one paraffin block.
Paraffin-embedded tissues were stained with hematoxylin and eosin (H&E) for routine histological examination. Briefly, the sections were de-waxed in xylene, re-hydrated through descending concentrations of ethanol and stained with hematoxylin (5 min) and eosin (2 min). An optical microscope (BX50, Olympus) was used for imaging.
RESULTS1) Development of lung cancer in orthotopic mice modelOn day 25 after inoculation of PC14PE6 cells, the whole thorax was occupied by a large lung cancer mass with scarce normal lung tissues. The largest tumor nodule was more than 1 cm in size. Microscopic examination of the lung tumor tissues demonstrated that normal alveolar structures of the lung had been destroyed by invasion of lung cancer cells, and the alveoli were filled with malignant cells (Fig. 1). When thoracic lymph nodes were dissected and examined microscopically, PC14PE6 malignant cells had efficiently infiltrated the parenchyma of the lymph nodes (Fig. 2).
We assessed the life span of orthotopic lung cancer model and investigated the life span in correlation with inoculated cell dose. Three doses of tumor cell inoculums (0.5×106/70 µl; 1×106/70 µl; 2×106/70 µl) were administered to the BALB/c mice by intrathoracic inoculation. Body weights of the animals were measured every day and an autopsy was performed immediately after natural death caused by lung cancer.
Nine mice in three groups (3 mice per group) developed lung to lung metastases and lung to intrathoracic lymph node metastases in the preliminary experiment. As shown in Fig. 3, the longest life span among three different cancer cell dosage-inoculated groups was 32 days with inocula of 0.5×106 cells in average and the shortest one was only 14 days with inocula 2×106 cells. These data indicated that the PC14PE6 cancer cells could induce rapid pathological propagation in the BALB/c nude mouse lung after inoculation. Despite rapid tumor development with heavy tumor burden in the animals, we could not detect any significant alterations in the body weight from most of animals before and after tumor inoculation. There were no statistical differences between control mice and the three tumor cell-inoculated groups (p≥0.05).
2) Pattern of spread of PC14PE6 cells in orthotopic animal modelWe further investigated the tumor development of two of the inoculation groups, 1×106 and 0.5×106. The first intrathoracic tumor was detected 7 days after inoculation in the 1×106 cancer cells-inoculated group (Table 1) when there were few cyst-like soft translucent masses in one of the three mice. By 10 days after inoculation, numerous tumor nodules, which had metastasized to lymph nodes and lung, were found upon thoracotomy. The average life span after inoculation was 25 days (13~39 days) in the 1×106 cancer cells inoculum group.
In the 0.5×106 cells inoculated group (Table 2), the first intrathoracic tumor was detected by thoracotomy 9 days after inoculation, which was longer than the time to detection in the 1×106 cancer cells-inoculated group. By 10 days after inoculation, the intrathoracic tumor mass was prominent with formation of solid mass and metastases to intrathoracic lymph nodes and lung. The life span was between 19 and 52 days with an average of 32 days. The representative tumor development after inoculation of PC14PE6 cancer cells is demonstrated in Fig. 4 and 5.
In order to investigate the pattern of spread to other distant organs, the brain, liver, kidney, muscle, spleen, adrenal gland, and lymph node on knee were removed and inspected with an optical microscope. None of the organs were invaded by PC14PE6 adenocarcinoma cells before death in this series. The body weights of the animals did not change significantly, and there were no statistical differences between the control and the experimental group (p≥0.05).
DISCUSSIONIn this study, the tumorigenicity percentage of mouse lung cancer using the intrathoracic inoculation method was more than 98%, which is higher than that of other orthotopic mouse lung cancer models using tail vein injections. The tumor cell propagation occurred rapidly after inoculation with solid mass formations after 10 days of inoculation. The life span of animals in our model was only one month when inoculated with 0.5×106 PC14PE6 cancer cells, whereas those in other models with intrathoracic inoculations of the same cell dose of NCI-H358, NCI-H226, NCI-H1299, A549, or NCI-H69 survive longer (8). A short life span in the mouse models will spare time to allow more efficient experimentation.
Since Paget proposed the original 'Seed and Soil' hypothesis in 1889, many in vivo models of the propagation of human tumors at orthotopic sites in athymic nude mice or SCID mice have been developed using renal cell carcinomas, brain tumors, colorectal carcinomas, liver cell carcinomas, pancreatic carcinomas, and lung carcinomas (11~14). The orthotopic mouse model of lung cancer was first developed by McLemore et al, who succeeded in growing human lung cancer cell lines in the bronchioloalveolar region by injecting intra-bronchial tumor cells (15). To date, there are three methods of establishing orthotopic lung cancer mouse models: intrathoracic injection of cancer cells (8); tail vein injection of cancer cells (16) and McLemore's transbronchial implantation method (15). The transbronchial implantation method is technically difficult (17), while the tail vein injection method has a low tumorigenicity percentage. Therefore, the most convenient and efficient method of generating an orthotopic lung cancer model in nude mice is the intrathoracic injection method.
Using this method, there were no distant metastases in other organs such as brain, liver, kidney, muscle, spleen, adrenal gland, or lymph node on knee after inoculation of PC14PE6 adenocarcinoma cells before death. This observation coincides with the previous study by Shiraga et al (18), which reported that the PC14PE6-induced tumor model differs from other models that developed multi-organ metastases. Because the PC14PE6 cancer cells propagate on a limited basis within the thorax, this model may be a convenient model for studying intrathoracic lung cancer without needing to monitor other organs.
Taken together, this orthotopic lung cancer model is an efficient cancer model with easy inoculation methods, rapid and high tumorigenicity, and simple monitoring methods for metastasis.
CONCLUSIONSThe reliable tumorigenicity time in the PC14PE6 adenocarcinoma cells inoculated into BALB/c nude mice were 10 days after intra-thoracic inoculation. The life span of the mice inoculated with the PC14PE6 cancer cells was 14 days with the injection of 2×106 cells; 22~25 days with 1×106 cells; 31~32 days with 0.5×106 cells, respectively. Metastasis of the PC14PE6 adenocarcinoma cells in this study was confined within thorax of mice. The reproducible and rapid tumorigenicity of the PC14PE6 adenocarcinoma cells intra-thoracially inoculated into BALB/c nude mice, shown in the present study, would allow researchers reliably test their hypothesis in the orthotopic mouse lung cancer xenograft model.
NotesThis work is supported by the project (0405-MN01-0604-0007) from Ministry of Health and Welfare, Korea. References1. Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1997;197:893–895. PMID: 887927
2. Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. PMID: 12568441
3. Pelleitier M, Montplaisir S. The nude mouse: a model of deficient T-cell function. Methods Achiev Exp Pathol. 1975;7:149–166. PMID: 1105061
4. Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J Cell Biochem. 1994;56:4–8. PMID: 7806591
5. Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. PMID: 2673568
6. Wilmanns C, Fan D, O'Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer. 1992;52:98–104. PMID: 1500231
7. Kuo TH, Kubota T, Watanabe M, Furukawa T, Kase S, Tanino H, et al. Site-specific chemosensitivity of human small-cell lung carcinoma growing orthotopically compared to subcutaneously in SCID mice: the importance of orthotopic models to obtain relevant drug evaluation data. Anticancer Res. 1993;13:627–630. PMID: 8391244
8. Onn A, Isobe T, Itasaka S, Wu W, O'Reilly MS, Hong WK, et al. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–5539. PMID: 14654533
9. Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–359. PMID: 10759402
10. Glinskii AB, Smith BA, Jiang P, Li XM, Yang M, Hoffman RM, et al. Viable circulating metastatic cells produced in orthotopic but not ectopic prostate cancer models. Cancer Res. 2003;63:4239–4243. PMID: 12874032
11. Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204–1208. PMID: 8439965
12. Fu X, Herrera H, Hoffman RM. Orthotopic growth and metastasis of human prostate carcinoma in nude mice after transplantation of histologically intact tissue. Int J Cancer. 1992;52:987–990. PMID: 1459741
13. Wang X, Fu X, Hoffman RM. A patient-like metastasizing model of human lung adenocarcinoma constructed via thoracotomy in nude mice. Anticancer Res. 1992;12:1399–1401. PMID: 1444197
14. Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. PMID: 1608975
15. McLemore TL, Liu MC, Blacker PC, Greqq M, Alley MC, Abbott BJ, et al. Novel intrapulmonary model for orthotopic propagation of human lung cancers in athymic nude mice. Cancer Res. 1987;47:5132–5140. PMID: 3621199
16. Goto H, Yano S, Zhang H, Matsumori Y, Ogawa H, Blakey D, et al. Activity of a new vascular targeting agent, ZD6126, in pulmonary metastases by human lung adenocarcinoma in nude mice. Cancer Res. 2002;62:3711–3715. PMID: 12097279
17. Mase K, Iijima T, Nakamura N, Takeuchi T, Onizuka M, Mitsui T, et al. Intrabronchial orthotopic propagation of human lung adenocarcinoma-characterizations of tumorigenicity, invasion and metastasis. Lung Cancer. 2002;36:271–276. PMID: 12009237
18. Shiraga M, Yano S, Yamamoto A, Ogawa H, Goto H, Miki T, et al. Organ heterogeneity of host-derived matrix metalloproteinase expression and its involvement in multiple-organ metastasis by lung cancer cell lines. Cancer Res. 2002;62:5967–5973. PMID: 12384564
Fig. 1Microscopic features of the mouse lung. H/E stain of paraffin-embedded sections of tumors within the mouse lung. The solid tumor mass (blue) was seen in the right image contrasting to the left normal one (magnification ×100). 
Fig. 2Microscopic features of the chest lymph node. H/E staining images of the paraffin-embedded sections of tumor developed within mouse lymph node in the chest. The center of the lymph node was occupied by PC14PE6 cancer cells (right) in contrast to the normal lymph node (left, magnification ×200). 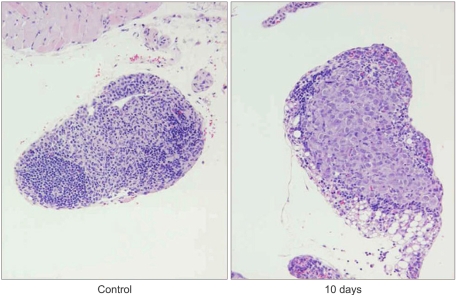
Fig. 3Average life span of three groups in the preliminary experiment. The mice were inoculated by 0.5×106; 1×106; and 2×106 PC14PE6 lung adenocarcinoma cells. 
Fig. 4Macroscopic features of the mouse thoracic cavity. PC14PE6 cells were injected into the thorax of nude mice, and experimental lung cancer was determined every 3 days beginning 4 days after inoculation. The tumor mass developed rapidly in the PC14PE6 cancer cell-inoculated nude mice. 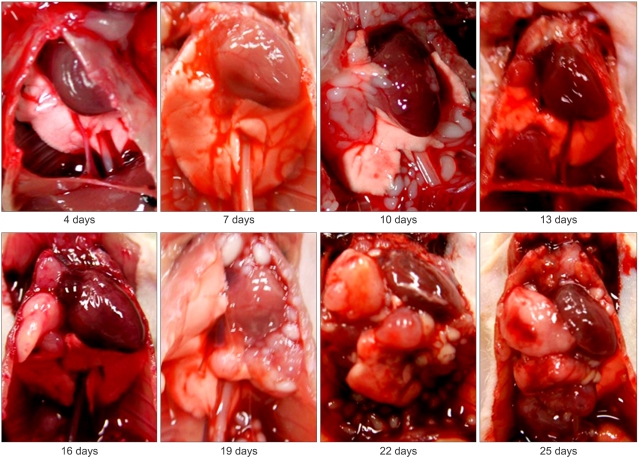
Fig. 5Microscopic features of the mouse lung. H/E stain of the paraffin-embedded sections of tumor developed within mouse lung. The solid tumor mass was seen in the PC14PE6 cancer cell-inoculated nude mice 10 days after inoculation and rapidly developed in the lung day by day (magnification ×40). 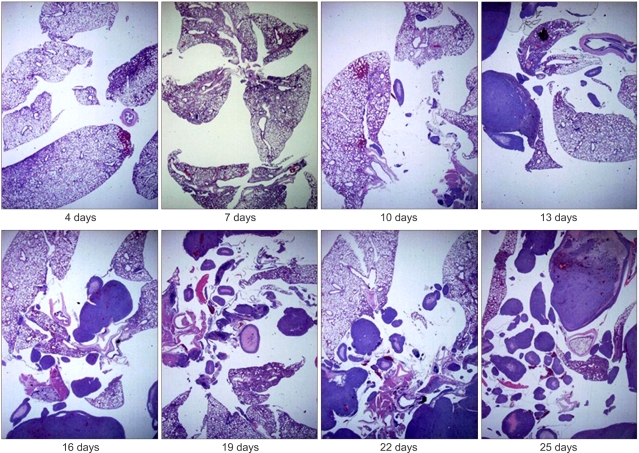
|
|
||||||||||||||||||||||||||||||||||||||