This article has been corrected. See ERRATUM: Prognostic Implications of Extranodal Extension in Relation to Colorectal Cancer Location. AbstractPurposeExtranodal extension (ENE) is closely associated with the aggressiveness of both colon and rectal cancer. This study evaluated the clinicopathologic significance and prognostic impact of ENE in separate populations of patients with colon and rectal cancers.
Materials and MethodsThe medical records of 2,346 patients with colorectal cancer (CRC) who underwent curative surgery at our institution between January 2003 and December 2011 were clinically and histologically reviewed.
ResultsENE was associated with younger age, advanced tumor stage, lymphovascular invasion (LVI), and perineural invasion (PNI) in both colon and rectal cancer. ENE rates differed significantly in patients with right colon (36.9%), left colon (42.6%), and rectal (48.7%) cancers (right vs. left, p=0.037; left vs. rectum, p=0.009). The 5-year disease-free survival (DFS) rate according to ENE status and primary tumor site differed significantly in patients with ENE-negative colon cancer (80.5%), ENE-negative rectal cancer (77.4%), ENE-positive colon cancer (68.6%), and ENE-positive rectal cancer (64.2%) (p<0.001). Multivariate analysis showed that advanced tumor stage, ENE, LVI, PNI, and absence of adjuvant chemotherapy were independently prognostic of reduced DFS in colon and rectal cancer patients.
ConclusionENE is closely associated with the aggressiveness of colon and rectal cancers, with its frequency increasing from the right colon to the left colon to the rectum. ENE status is a significant independent predictor of DFS in CRC patients irrespective of tumor location. ENE might be more related with distally located CRC.
IntroductionExtranodal extension (ENE) of metastatic lymph nodes (LNs), which is indicated the extension of tumor cells beyond the nodal capsule into the perinodal adpose tissue, is an important prognostic factor in patients with several types of malignancies, including stomach, thyroid, bladder, and breast cancer [1-8]. ENE is also included in the American Joint Committee on Cancer (AJCC) eighth staging system for patients with penile cancer, vulvar cancer, and head and neck squamous cell carcinoma [9].
In contrast, ENE in patients with colorectal cancer (CRC) is not yet considered as TNM staging system. Nevertheless, a recent meta-analysis reported that the presence of ENE was an important prognostic factor in patients with node-positive CRC. However, this study, analyzed the prognostic effect of ENE in patients with CRC when considering colon and rectal cancers as only one entity.
It is unclear whether colon cancer and rectal cancer are biologically different malignancies or are the same malignancy at different locations. Accumulating evidence provide that colon and rectal cancer differed not only in etiologies, risk factors, anatomic site but in embryological origin, function, and metastatic patterns [10-12], suggesting that the prognostic implications of ENE should be investigated separately in patients with colon and rectal cancer [13]. Furthermore, right- and left-sided colon cancers may represent distinctive and different disease entities (two colon concept) [10,14]. Unfortunately, despite the difference according to location of CRC, little attention has been paid to the histologic feature of ENE. This study therefore assessed the clinicopathologic significance and prognostic impact of ENE in large, separate populations of patients with colon and rectal cancer.
Materials and Methods1. PatientsThe medical records of 2,346 patients with CRC having metastatic LNs who underwent surgery at Asan Medical Center (Seoul, Korea) between January 2003 and December 2011 were retrieved from the center’s database, and their clinical and histological characteristics were reviewed. All patients with node-positive CRC who had histologically proven adenocarcinoma and underwent curative resection (R0) were included. Patients with hereditary CRC (familial adenomatous polyposis and hereditary nonpolyposis CRC), multiple CRCs, and stage IV CRC were excluded. Also, we excluded were patients who received preoperative chemoradiotherapy or chmotherapy because tumor pathology may have been affected by preoperative treatment.
2. EvaluationBefore surgery, all patients underwent a staging workup, including colonoscopy, chest radiography, computed tomography (CT) of the abdomen and pelvis, and measurement of serum carcinoembryonic antigen (s-CEA) concentrations. Some patients also underwent positron emission tomography (PET) scanning and single contrast-enhanced magnetic resonance imaging (MRI) of the rectum and liver. s-CEA was measured by enzyme immunoassay (ELISA-2-CEA kit, CIS Bio International, Marcoule, France), with the normal concentration defined as ≤ 6 ng/mL. Tumors were pathologically staged in accordance with the AJCC cancer staging manual, eighth edition [9].
3. Histologic evaluationThe ENE status of all specimens was examined by two pathologists (J. Kim and Y. Park) and the final diagnosis was based on intradepartmental consultations with staff specialized in CRC. ENE was defined as cancer cells infiltrating the extranodal adipose tissue beyond the capsule of the LN (Fig. 1A). Tumor deposits (Fig. 1B) and tumor cells outside the LNs, continuous with the primary tumor (Fig. 1C), or confined to endolymphatic spaces (Fig. 1D) were not considered ENE. A tumor was considered ENE-positive when one or more of the metastatic LNs showed ENE, as described [15].
The distance between the tumor and the circumferential resection margin (CRM) was measured in millimeters. If CRM was found within 1mm of the tumor, we defined the positive CRM.
4. Adjuvant chemotherapyOf the 2,346 patients in the study cohort, 2,151 (91.7%) received postoperative chemotherapy. Separate assessment of the 1,363 colon cancer patients showed that 1,273 (93.4%) received postoperative chemotherapy, including 105 (7.7%) who received 5-fluorouracil, 530 (38.9%) who received capecitabine, 517 (37.9%) who received oxaliplatin, 53 (3.9%) who received an oral pyrimidine analogue or oral 5-fluorouracil, and 68 (5.0%) who received chemotherapy at another hospital. The remaining 90 patients (6.6%) did not receive adjuvant chemotherapy.
Of the 983 rectal cancer patients, 878 (89.3%) received postoperative chemotherapy, including 303 (30.8%) who received 5-fluorouracil, 331 (33.7%) who received capecitabine, 108 (11.0%) who received oxaliplatin, 44 (4.5%) who received an oral pyrimidine analogue or oral 5-fluorouracil, and 92 (9.4%) who received chemotherapy at another hospital. The remaining 105 patients (10.7%) did not receive adjuvant chemotherapy. In addition, 382 (38.9%) rectal cancer patients received postoperative radiotherapy. Usually, our center recommended the postoperative radiotherapy for stage II or stage III mid to lower rectal cancer patients. For data analysis, patients who received intravenous 5-fluorouracil–based or capecitabine-/oxaliplatin-based chemotherapy were regarded as receiving complete adjuvant chemotherapy.
5. Follow-upStandardized postoperative follow-up included clinical examinations, complete blood counts, blood chemistry tests, measurements of s-CEA levels, and chest radiography. Patients were followed up every 3 months for the first 2 postoperative years and every 6 months thereafter. Patients also underwent abdominal and pelvic CT scans every 6 months. Colonoscopy was performed within 1 year of surgery and then once every 2-3 years. If recurrence was suspected, patients were evaluated by CT, MRI, and/or PET scanning. Recurrence was diagnosed pathologically (by surgical resection or biopsy) and/or radiologically.
6. Statistical analysisCategorical variables were compared using chi-square tests, and continuous variables were compared using independent sample t tests. The Kaplan-Meier method was used to compare disease-free survival (DFS). DFS was defined as the time from the date of the primary tumor surgical resection to the time of disease recurrence which was diagnosed pathologically or radiologically or death. Univariate and multivariate analyses of factors associated with DFS rates were performed using Cox proportional hazard regression models to estimate hazard ratios and 95% confidence intervals. All statistical tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 21.0 for Windows (IBM Corp., Armonk, NY).
Results1. Patient characteristicsOf the 2,346 patients included in this study, 1,363 (58.1%) were diagnosed with colon cancer and 983 (41.9%) with rectal cancer. A total patient group included 1,389 male (59.2%) and 957 female (40.8%). The mean age of these patients was 60±11 years (median age, 61; range, 19 to 89 years). All patients received curative intent surgery, with evidence of pathologic stage IIIA in 280 (11.9%), stage IIIB in 1,711 (72.9%), and stage IIIC in 355 (15.1%). ENE in a metastatic LN was detected in 551 (40.4%) colon cancer patients, including 195 (36.9%) patients with right colon cancer, 356 (42.6%) with left colon cancer, and 479 (48.7%) with rectal cancer (right vs. left, p=0.037; left vs. rectum, p=0.009). The mean follow-up interval was 60±32 months. The 5-year DFS rate in patients with right and left colon cancer differed significantly by ENE status, being highest in patients with ENE-negative left colon cancer (80.8%), followed by patients with ENE-negative right colon cancer (80.0%), ENE-positive left colon cancer (70.1%), and ENE-positive right colon cancer (65.6%) (p < 0.001) (Fig. 2A). However, the 5-year DFS rate in patients with colon and rectal cancer differed significantly by tumor site and ENE status, being 80.5%, 77.4%, 68.6%, and 64.2% in patients with ENE-negative colon cancer, ENE-negative rectal cancer, ENE-positive colon cancer, and ENE-positive rectal cancer, respectively (p < 0.001) (Fig. 2B).
2. Clinicopathologic characteristics according to ENE status in colon and rectal cancer patientsThere were no significant differences in sex, s-CEA level, and histologic type between ENE-positive and ENE-negative colon and rectal cancer patients. However, ENE was more frequent in younger patients and those with higher T category, higher N category, lymphovascular invasion (LVI), and perineural invasion (PNI). ENE was more frequent in patients with left than right colon cancer (Table 1).
3. Prognostic factors of DFS in colon cancer patientsThe 5-year DFS rate was significantly lower in ENE-positive than ENE-negative colon cancer (68.6% vs. 80.5%, p < 0.001). Univariate analysis showed that elevated s-CEA, pathologic stage, presence of ENE, presence of LVI, presence of PNI, and lack of adjuvant chemotherapy were associated with poorer DFS. Multivariate analysis showed that pathologic stage, presence of ENE, presence of LVI, presence of PNI, and lack of adjuvant chemotherapy were significant independent predictors of DFS (Table 2).
4. Prognostic factors of DFS in rectal cancer patientsThe 5-year DFS rate was also significantly lower in ENE-positive than ENE-negative rectal cancer patients (64.1% vs. 77.4%, p < 0.001). Univariate analysis showed that elevated s-CEA, pathologic stage, presence of ENE, presence of LVI, and presence of PNI, positive of CRM and lack of adjuvant were associated with poorer DFS. Multivariate analysis showed that pathologic stage, presence of ENE, presence of LVI, presence of PNI, and lack of adjuvant chemotherapy were significant independent predictors of DFS (Table 2).
5. Recurrence patterns according to tumor location and ENE statusRecurrence patterns in colon cancer patients were unrelated to ENE status. LN recurrence was slightly higher in ENE-positive than in ENE-negative patients, but the difference was not statistically significant. In rectal cancer patients, however, LN and multiple route recurrences (e.g., hematogenous and LN, or hematogenous and peritoneal seeding) were significantly more frequent in ENE-positive than in ENE-negative patients (Table 3).
DiscussionThis study investigated the clinical implications of ENE separately in patients with colon and rectal cancer who were treated at a single center during a 9 year period. We show that ENE is significantly associated with worse DFS in large cohort of patients with colon and rectal cancer, respectively, independent of the standard prognostic and predictive factors: pathologic stage, LVI, and PNI. Furthermore, ENE in both colon and rectal cancer significantly correlated with younger age, higher T category, higher N category, and occurrences of LVI and PNI. In general, patients with rectal cancer have a poorer prognosis than those with colon cancer [16]. However, we also found that 5-year DFS rates were poorer in ENE-positive colon cancer than in ENE-negative rectal cancer patients, being 77.4% in patients with ENE-positive colon cancer and 68.6% in patients with ENE-negative rectal cancer (p < 0.001). Taken together, these findings suggest that ENE is one of important indicators of prognosis and ENE can be considered a hallmark of tumor aggressiveness. In general, patients with left-sided CRC are younger than those with right-sided CRC [14]. As ENE is more frequent in the left-sided than the right-sided CRC, ENE may be associated with younger age.
ENE in metastatic LNs was found to be a well-known negative prognostic factor in patients with many other tumor types. Recently, ENE was reported to be a poor prognostic factor in patients with gastrointestinal tract cancers including esophageal [17], stomach [1], and colorectal [15,18] cancers.
CRC can be subdivided by site, namely, the proximal colon, distal colon, and rectum [10,14]. These sites differ in embryological origin, morphology, and physiology [11,14,19], and histological differences have been observed in proximal and distal CRC [20]. Epidemiologically, site-specific CRCs differ according to sex and age [21]. Consequently, primary rectal and colon cancers present with different recurrence patterns and prognosis, and may require different treatment modalities.
Genomic determinants are important predictors of tumor development and progression [22], and differ by tumor location. Two main syndromes resulting from germline mutations play a role in the occurrence of CRCs. In patients with familial adenomatous polyposis, tumors occur predominantly in the distal colon (~60%) and rectum (~25%) [23]. In patients with hereditary nonpolyposis colorectal cancer, however, tumors develop predominantly in the proximal colon (~55%) and rectum (~15%). Chromosomal instability has been associated with 60%-70% of CRCs, more frequently in the distal than in the proximal colon [24]. By contrast, the frequencies of CpG island methylator phenotype–high, high frequency of microsatellite instability, and BRAF mutations have been reported to increase in a retrograde manner from the rectum to the ascending colon [10]. Therefore, Yamauchi et al. insisted that the frequencies of molecular pathologic changes in CRC evolve gradually through bowel location, rather than after abruptly at the splenic flexure and rectosigmoid junction (continuum hypothesis) [10]. These findings suggested a close association between molecular subtype and site-specificity. Interestingly, this study showed that ENE rates increased significantly from patients with right colon cancer (36.9%) to left colon cancer (42.6%) to rectal cancer (48.7%) (right vs. left, p=0.037; left vs. rectum, p=0.009). These facts were that ENE was a common aggressive feature of CRC; however, it might be more related with distal part CRC. Considering continuum hypothesis, frequency of ENE increases from the right colon to the left colon to the rectum. However, genomic and molecular level investigation is required to verify our assertions.
Multivariate analyses showed that advanced tumor stage, ENE, LVI, PNI, and lack of adjuvant chemotherapy were independent poor prognostic factors for DFS in patients with colon cancer and rectal cancer. Pathologic tumor stage is a universally powerful prognostic factor [9], along with LVI or PNI [25,26]. Adjuvant chemotherapy significantly improves DFS and overall survival of patients with stage III CRC [27]. Our results showed that the prognostic impact of ENE in patients with colon and rectal cancer was same. However, we found that patients with ENE-negative rectal cancer had a better prognosis than those with ENE-positive colon cancer. In general, colon cancer is known to have a better survival rate than rectal cancer, but the survival rate of colon and rectal cancer can be reversed according to ENE status. Therefore, ENE status as well as tumor location may be an important prognostic indicator.
The limitations of this study include its retrospective design and its inclusion of patients who were treated at a single center, restricting the ability to generalize from study results. To our knowledge, however, this study is the first to report the prognostic effect of ENE separately in patients with colon cancer and rectal cancer by analyzing a relatively large patient cohort.
In conclusion, the occurrence of ENE is closely related to the aggressiveness of both colon and rectal tumors. ENE status is a significant independent prognostic factor regardless of tumor location. However, ENE might be more related with distally located CRC.
AcknowledgmentsThis work was supported by a grant (to J.C. Kim) from the Korea Research Foundation (2016R1E1A1A02919844), Ministry of Science, ICT, and Future Planning, Republic of Korea.
Fig. 1.Representative examples of lymph node metastasis patterns. (A) Tumor cells invading fat tissue (arrows) beyond the boundary of the lymph node (dashed line). Extranodal extension positive (×1.25 objective lens, scale bar=2 mm). (B) Tumor deposits with an invasive margin separate from the primary tumor. Extranodal extension negative (×1.25 objective lens, scale bar=2 mm). (C) Tumor cells outside the lymph node but in continuum with the primary tumor. Extranodal extension negative (×4 objective lens). (D) Tumor cells outside the lymph node but confined to endolymphatic spaces (arrow). Extranodal extension negative (×10 objective lens, scale bar=200 μm). 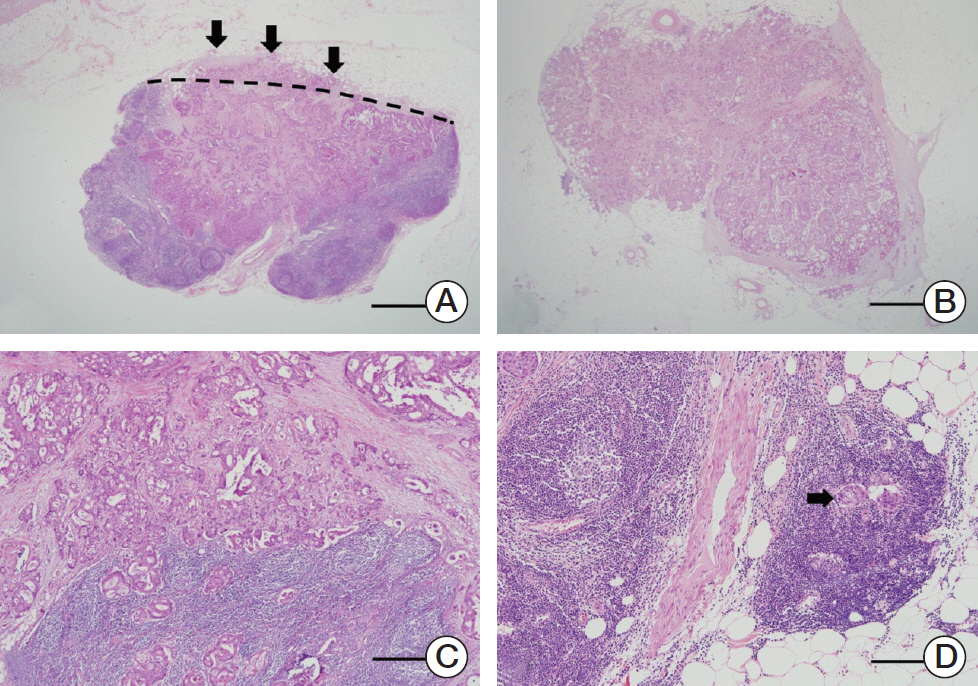
Fig. 2.(A) Disease-free survival curves in patients with colon cancer according to tumor location (right colon vs. left colon) and extranodal extension (ENE) status (n=1,363). (B) Disease-free survival curves in patients with colon and rectal cancer according to tumor location (colon vs. rectum) and ENE status (n=2,346). 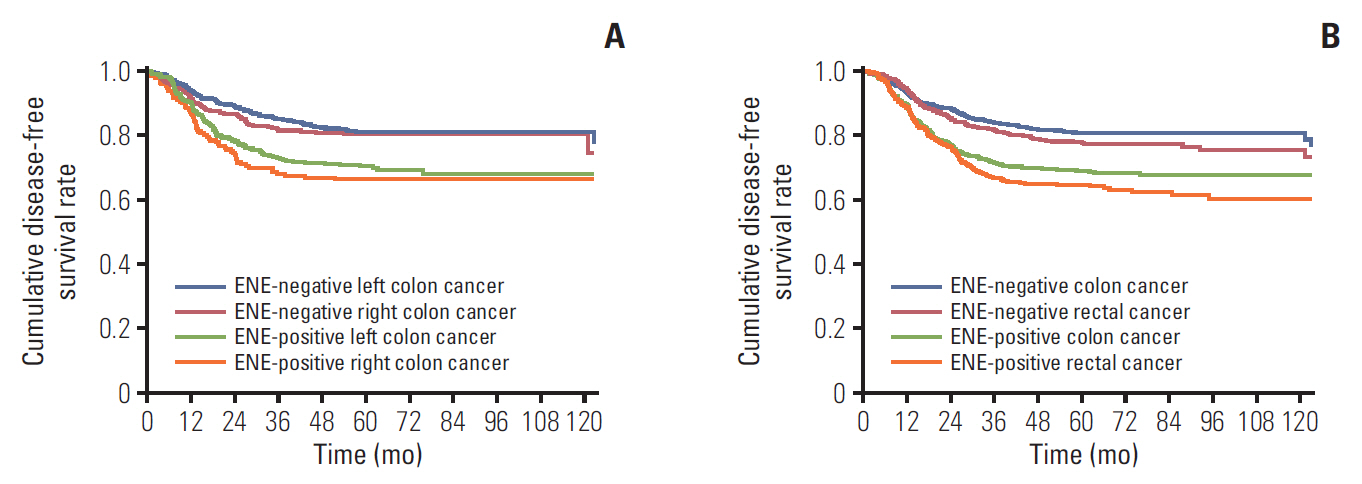
Table 1.Clinicopathologic characteristics of patients with colorectal cancer with and without extranodal extension (n=2,346) Values are presented as number (%) or mean±standard deviation unless otherwise indicated. ENE, extranodal extension; s-CEA, serum carcinoembryonic antigen; RC, right colon; LC, left colon; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell; Muc, mucinous; LVI, lymphovascular invasion; PNI, perineural invasion; CTx, chemotherapy. Table 2.Univariate and multivariate analysis of factors prognostic of DFS in patients with node-positive colon and rectal cancer DFS, disease-free survival; CI, confidence interval; s-CEA, serum carcinoembryonic antigen; WD, well-differentiated; MD, moderately differentiated; PD, poorly differentiated; Muc, mucinous; SRC, signet ring cell; RC, right colon; LC, left colon; ENE, extranodal extension; LVI, lymphovascular invasion; PNI, perineural invasion; CTx, chemotherapy; CRM, circumferential resection margin. Table 3.Recurrence pattern according to tumor location and ENE status References1. Lee IS, Park YS, Ryu MH, Song MJ, Yook JH, Oh ST, et al. Impact of extranodal extension on prognosis in lymph node-positive gastric cancer. Br J Surg. 2014;101:1576–84.
2. Suh S, Pak K, Seok JW, Kim IJ. Prognostic value of extranodal extension in thyroid cancer: a meta-analysis. Yonsei Med J. 2016;57:1324–8.
3. Fajkovic H, Cha EK, Jeldres C, Robinson BD, Rink M, Xylinas E, et al. Extranodal extension is a powerful prognostic factor in bladder cancer patients with lymph node metastasis. Eur Urol. 2013;64:837–45.
4. Nottegar A, Veronese N, Senthil M, Roumen RM, Stubbs B, Choi AH, et al. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory meta-analysis. Eur J Surg Oncol. 2016;42:919–25.
5. Zhang ZL, Yu CP, Liu ZW, Velet L, Li YH, Jiang LJ, et al. The importance of extranodal extension in penile cancer: a meta-analysis. BMC Cancer. 2015;15:815.
6. Lee YC, Wu CT, Kuo SW, Tseng YT, Chang YL. Significance of extranodal extension of regional lymph nodes in surgically resected non-small cell lung cancer. Chest. 2007;131:993–9.
7. van der Velden J, van Lindert AC, Lammes FB, ten Kate FJ, Sie-Go DM, Oosting H, et al. Extracapsular growth of lymph node metastases in squamous cell carcinoma of the vulva. The impact on recurrence and survival. Cancer. 1995;75:2885–90.
8. Luchini C, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, et al. Significance of the prognostic stratification of extranodal extension in colorectal cancer. Ann Oncol. 2016;27:1647.
9. Edge SB, Greene FL, Byrd DR, Brookland RK, Waashington MK, Gershenwald JE, et al. AJCC cancer staging manual. 8th edNew York: Springer; 2017.
10. Yamauchi M, Lochhead P, Morikawa T, Huttenhower C, Chan AT, Giovannucci E, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7.
11. Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41:671–9.
12. Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–42.
13. Huang Q, Yang H. Prognostic impact of extra-nodal extension on colon and rectal cancer should be investigated separately. Ann Oncol. 2016;27:956–7.
15. Kim CW, Kim J, Yeom SS, Lee JL, Yoon YS, Park IJ, et al. Extranodal extension status is a powerful prognostic factor in stage III colorectal cancer. Oncotarget. 2017;8:61393–403.
16. Wang B, Yang J, Li S, Lv M, Chen Z, Li E, et al. Tumor location as a novel high risk parameter for stage II colorectal cancers. PLoS One. 2017;12:e0179910
17. Luchini C, Wood LD, Cheng L, Nottegar A, Stubbs B, Solmi M, et al. Extranodal extension of lymph node metastasis is a marker of poor prognosis in oesophageal cancer: a systematic review with meta-analysis. J Clin Pathol. 2016;69:956–91.
18. Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol. 2016;27:42–8.
19. Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6.
20. Ghazi S, Lindforss U, Lindberg G, Berg E, Lindblom A, Papadogiannakis N, et al. Analysis of colorectal cancer morphology in relation to sex, age, location, and family history. J Gastroenterol. 2012;47:619–34.
21. Butcher D, Hassanein K, Dudgeon M, Rhodes J, Holmes FF. Female gender is a major determinant of changing subsite distribution of colorectal cancer with age. Cancer. 1985;56:714–6.
22. Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27.
23. Bertario L, Russo A, Sala P, Eboli M, Radice P, Presciuttini S, et al. Survival of patients with hereditary colorectal cancer: comparison of HNPCC and colorectal cancer in FAP patients with sporadic colorectal cancer. Int J Cancer. 1999;80:183–7.
24. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
25. Alotaibi AM, Lee JL, Kim J, Lim SB, Yu CS, Kim TW, et al. Prognostic and oncologic significance of perineural invasion in sporadic colorectal cancer. Ann Surg Oncol. 2017;24:1626–34.
|
|
|||||||||||||||||||||||||||||||||||||||||