CASE REPORT
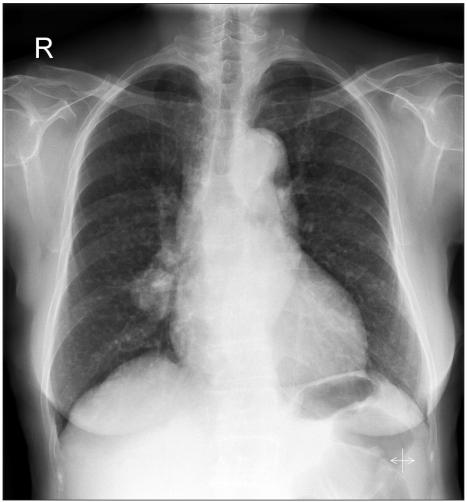
A 73-year-old woman presented in the clinic with multiple lung nodules. Neither she nor her husband had ever smoked. An initial chest x-ray showed a main lung mass in the right middle lobe with variable sized nodules in both lungs (Fig. 1). Bronchoscopy did not show a definite endobronchial lesion. A CT-guided needle biopsy of the right middle lobe mass showed moderately differentiated adenocarcinoma. The patient was treated with 4 cycles of gemcitabine and cisplatin between September and December 2003; the disease remained stable after 2 cycles of chemotherapy.
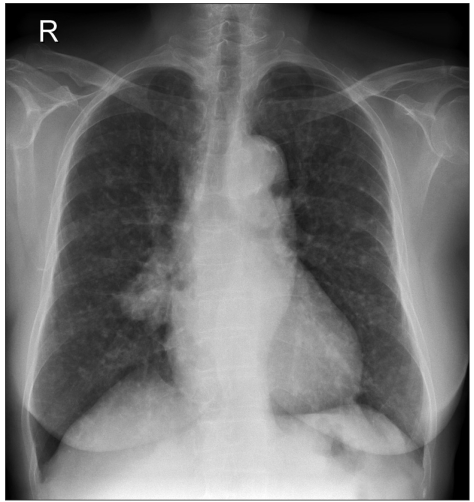
A chest x-ray taken on February 2004 showed an increased size and number of nodules in both lungs (Fig. 2). However, the patient remained free of any respiratory or constitutional symptoms. On March 2004, the patient was started on gefitinib, 250 mg a day, and continued this treatment until September 2004 for 6 months. Gefitinib treatment resulted in a remarkable response with a decrease in the size and number of bilateral lung nodules (Fig. 3). The patient tolerated the treatment with only grade 1 skin toxicity. After 6 months of treatment, gefitinib was discontinued due to disease progression.
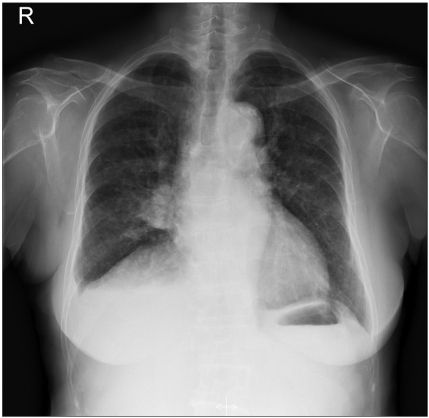
The patient refused further therapy despite of progression of the lung lesions (Fig. 4). On April 2005, when erlotinib became available in Korea, the patient was started on erlotinib, 150 mg a day. There was a remarkable tumor response that qualified for partial response after erlotinib therapy again (Fig. 5).
Grade 1 skin toxicity developed after the administration of erlotinib. The patient was able to take erlotinib for 11.5 months until disease progression was again noted.
Eventually, the patient developed multiple brain metastases on May 2006 and died of lung cancer with accompanying brain metastases. She lived for 33 months after the diagnosis of stage IV adenocarcinoma of the lung and for 26 months after the administration of gefitinib.
DISCUSSION
On March 2005, the U.S. Food and Drug Administration (FDA) withdrew the approval of gefitinib based on the results of the ISEL Study (1) that compared the use of gefitinib with placebo. While the ISEL study failed to demonstrate any of survival advantages of using gefitinib over placebo, high response rates were observed in a sugbgroup of patients: those who never-smoked, women, those with adenocarcinoma and those of Asian origin. A survival benefit was observed in patients who were never smokers and those who are of Asian origin (1). In another study conducted in Korea, patients with an EGFR mutation showed a higher response rate to gefitinib treatment with a significant survival benefit (2). Although gefitinib was withdrawn in the US market and is rarely used in Europe, gefitinib is still used in many of Asian countries including Korea, Taiwan, China and Japan. Its continued use is justified based on data of a subset analysis of the ISEL study and other studies which showed not only a high response rate but also a survival benefit in Asian women who never smoked and who demonstrate adenocarcinoma pathology (3).
A similar, but not identical EGFR tyrosine kinase inhibitor, erlotinib was approved November 2004 by the US. FDA for use in patients who had failed on a previous chemotherapy regimen. A randomized, placebo-controlled Phase III trial (BR.21) (4) demonstrated a survival advantage for treatment with erlotinib in all patient subsets with the greatest benefit shown in never-smokers with adenocarcinoma and those of Asian origin. In multiple logistic-regression analyses, never-smokers, the presence of adenocarcinoma, and EGFR expression were associated with erlotinib responsiveness (4). However, to our best knowledge, there have been few case reports describing the outcome of sequential treatment with two different EGFR tyrosine kinase inhibitors.
Our case may be the first ever reported in Korea that responded sequentially to erlotinib therapy following initial therapy with gefitinib.
Viswanathan and colleagues (5) reported a lack of response to erlotinib after progression on gefitinib therapy in 4 women who responded to gefitinib initially and in 1 man who was refractory to treatment with gefitinib. However, Garfield (6) reported a response to treatment with erlotinib in a gefitinib refractory man. Choong and colleagues (7) reported a response to gefitinib therapy in an erlotinib refractory Japanese-American woman who had never smoked, and who had a novel double L858R+E884K somatic mutation in the EGFR gene. An explanation for a response in this patient was not given.
Our experience suggests that erlotinib therapy could be administered to patients who develop progressive disease after initial therapy with gefitinib that has produced an initial response.