AbstractPurposeBronchial wash fluid may be a useful for detecting lung cancer. To increase the detection rates, we performed molecular analysis with using MAGE A1-6 and SSX4 RT-PCR on bronchial wash fluid specimens.
Materials and MethodsWe obtained 57 lung cancer tissue specimens by bronchoscopic biopsy and 131 bronchial washes from 96 patients with lung cancer and 35 patients with benign lung diseases. The MAGE A1-6 and SSX4 gene expressions were investigated in the cancer tissue specimens and bronchial wash fluids. We evaluated the positive detection rates of these methods according to the cytology results and the clinical findings.
ResultsFor the cancer tissue specimens and the bronchial wash fluid, the positive detection rate of MAGE or SSX4 was 91.2% and 75.0%, respectively. Combined MAGE and SSX4 PCR and cytology tests showed an 83.3% detection rate for the bronchial wash fluid. From bronchial washes of patients with benign lung diseases, the positive rates of using MAGE or SSX4 was 11.4%. In the bronchial wash fluid of lung cancer patients, 66.7% of the peripheral cancers were detected by MAGE or SSX4, while examination with cytology did not detect any peripheral lung cancer.
INTRODUCTIONIn order to improve the early detection rate of lung cancer, we designed melanoma-associated antigen (MAGE) A1-6 common primers (1) that could detect the markers MAGE A1 to A6 simultaneously. MAGE is not expressed in normal tissues, except in the testis and placenta (2). A MAGE expression is generally considered to be a tumor specific event (3). Performing MAGE RT-PCR with using MAGE A1-6 common primers has been used to determine the lung cancer detection rates in cancer specimens from random sputum, induced sputum, BAL and pleural fluids (4). Among all the available respiratory specimens, induced sputum might be the best specimen for lung cancer screening because it is easily obtained. Therefore, we focused our attention on induced sputum as being a practical screening specimen for detecting lung cancer; however, the rates for detecting lung cancer with using sputum samples were variable depending on the specimen quality.
Although sampling of bronchial wash fluid is relatively invasive, the resulting specimens may be more dependable and they contain more lung cancer cells than are found in induced sputum samples (5). There have been many molecular studies on lung cancer detection with using bronchial lavage fluid (6~ 10), but these studies had limited success finding a simple, convenient and reliable method that could be easily adapted to the clinical laboratory.
Synovial sarcoma on the X chromosome gene (SSX) is a cancer testis antigen gene that's expressed in cancer tissues, but not in normal tissues except for the thyroid and testis (11~13). SSX has nine subtypes, SSX1 to SSX9 (11); among the different subtypes, SSX4 has been reported to show a high expression (35%) in lung cancer (14). The combined use of MAGE A1-6 and SSX4 might increase the rate of detecting cancer.
We performed molecular analysis with using MAGE A1-6 and SSX4 RT-PCR and we compared the results to that of a conventional cytological examination. In addition, we determined the sensitivity and specificity of MAGE A1-6 and SSX4 for detecting lung cancer in bronchial wash fluid.
MATERIALS AND METHODSTo evaluate the rates MAGE and SSX4 are expressed from cancer tissues, we obtained 57 lung cancer tissues via bronchoscope biopsy. The cancer tissue specimens included 5 with adenocarcinoma, 11 with small cell carcinoma, 39 with squamous cell carcinoma and 2 with other cancers: 1 large cell and 1 mucoepidermoid cancer, respectively. For the clinical application, another 131 bronchial washes were randomly obtained from the patients who visited Yeungnam University Hospital for pulmonary complaints. These patients were finally diagnosed with using the CT or bronchoscopic findings, and the bronchoscopic or percutaneous needle biopsy results; 96 patients had lung cancer and 35 patients had benign lung disease. The average age of the patients with lung cancer and benign lung diseases was 64.6±8.8 and 59.5±18.2 respectively, and the male to female ratio was 5.3:1 and 1.2:1 for each group, respectively.
The collected fluids (6 ml) were divided into two tubes: one for cytological examination and one for molecular analysis. The cancer tissues were immediately added to micro-tubes that contained 1.0 ml TRI reagent (Molecular Research Center, Cincinnati, OH); the wash fluids for molecular analysis were added to 15 ml conical tubes that contained RNA preservation solution (IC&G Co, Korea). The specimens were stored at -70℃ until RNA extraction was done. RNA extraction was performed using the TRI method for tissues; an RNA extraction kit (IC&G Co, Korea) was used for the bronchial washes. The total RNA (1 µg) was reverse transcribed with using an ImProm-II Reverse transcription system (Promega, Madison, WI). The MAGE and SSX4 expressions were investigated by performing nested PCR with using MAGE A1-6 common and SSX4 specific primers, and these primers were designed by the investigators. The integrity of obtained cDNA was tested by amplifying the GAPD transcripts:; the sense primer was 5'-TCG GAG TCA ACG GAT TTG GTC GTA, and the antisense primer, 5'-CAA ATG AGC CCC AGC CTT CTC CA. The sequences from the MAGE A1-6 common primers have been described previously (1). The primers for SSX4 nested PCR were as follows: sense primer, 5'-GTC ACC CTC CCA CCT TTC AT; antisense primer, 5'-TCA CCA CGT TCT GCT TCT CAT CAT; nested sense primer, 5'-ATG ACT TTC GGC AGC CTC CAG A; nested antisense primer, 5'-CTT CTC ATC ATG GGC ATG TGT CAT. The primer sets for the nested PCR produced DNA products that were 426 bp and 311 bp, respectively. For the individual PCR reactions, 4 µl of cDNA were amplified with using gene-specific oligonucleotides (10 pmol per 50 µl reaction with using Eppendorf Hot- MasterMix (Eppendorf, Germany). The cytology evaluations were performed with using Papanicolaou stain and the cytology was analyzed by one pathologist.
The positive detection rates were evaluated according to the cell type, the cancer stage and the location. The cancer stages were classified according to the American Joint Committee on Cancer, and the tumor locations were categorized as central or peripheral tumor according to the visibility of the cancer with using a 5.8 mm fiberoptic bronchoscope (Olympus, Japan).
The study design was approved by the IRB at Yeungnam University Hospital and informed consents were obtained from all the patients.
The differences of the lung cancer detection rates were analyzed according to the detection methods, and the clinical findings were analyzed by the chi square test with using SPSS software (version 14.0; SPSS, Chicago, IL). p values <.05 were considered significant.
RESULTS1) Sensitivity and specificityAmong the 57 cancer tissues, the average expression rates for MAGE and SSX4 were 89.5% and 29.1%, respectively. The MAGE expression rates varied from 80% to 90.9%, while the SSX4 expression rates varied from 9.1% to 40.0% according to the cell type. The positive rate of lung cancer detection with using the MAGE or SSX4 gene expression was 91.2% (Table 1).
For the 96 bronchial washes from the lung cancer patients, the positive rates for the cytology examination and the MAGE and SSX4 expressions were 50.0%, 69.8% and 30.9%, respectively (Fig. 1); the positive lung cancer detection rate for using the MAGE or SSX4 expression was 75.0%. Based on cell type, the cytology exam and the MAGE expression showed the highest detection rates for squamous cell carcinoma, while the SSX4 expression had the highest detection rate for adenocarcinoma. The MAGE testing demonstrated a significantly higher detection rate compared to the cytology exam, and this was especially so for squamous cell cancer. With using both molecular markers and a cytological examination, the detection rate for lung cancer was significantly increased from 50.0% for cytology alone, to 83.3% (Table 2).
For the 35 bronchial wash samples from the patients with benign lung diseases, four cases (11.4%) showed a positive MAGE expression in specimens from patients with tuberculosis or other lung diseases, while SSX4 was expressed only in one of tuberculosis specimens. Though statistically insignificant, the expression rate for SSX4 was lower than that of MAGE in bronchial wash fluids from patients with benign lung diseases (Table 3).
2) Positive detection rates based on T stage and cancer locationsFor the cases with T1 and T2 stage cancers, the positive rate of using cytology, or the MAGE or SSX4 expressions was 57.6, 66.7 and 27.3%, respectively (Fig. 2). The combination of using molecular and cytology tests revealed a significantly higher detection rate (84.8%) than that of performing cytological examination alone. In cases with T3 and T4 stage cancers, the positive rates of using cytology and the MAGE and SSX4 expressions was 46.7, 73.3 and 32.6%, respectively. The combination of using molecular and cytology tests revealed a significantly higher detection rate (82.2%) than that of using cytological examination alone. For peripheral cancer locations, cytology tests did not detect any lung cancer; however, MAGE or SSX4 tests detected 66.7% of these lung cancers. Each of the molecular tests was significantly more sensitive than the cytology tests (p<0.01). For central cancer locations, the cytology test detected 63.2% of the lung cancer cases, while the MAGE or SSX4 tests could detect 77.6% of the lung cancer cases. With combining tests for the MAGE and SSX4 expressions and the cytology, the positive detection rate increased up to 88.2% (Fig. 3).
DISCUSSIONCompared to sputum, bronchial wash fluid contains many more lung cancer cells; thus, it might be expected that the detection rate for lung cancer would usually be higher with using bronchial wash specimens compared to sputum (5). In the case of a peripheral lung cancer, low dose spiral CT, PET and bronchoscopy are the currently used modalities to screen for peripheral lung cancer; however, fine needle aspiration and cytological examination are still required for confirming the diagnosis (15). Yet needle aspiration has the risk of cancer dissemination (16,17); in addition, the cytological diagnosis may be equivocal (18,19). Bronchoalveolar lavage fluid may provide an adequate specimen for detecting lung cancer (20,21), but bronchial wash fluid is easier to obtain for both the patients and the pulmonologist. In prior studies, cytology evaluations have been reported to detect lung cancer in 17.7% to 80.5% of the cases when using bronchial wash specimens (5,18,19,21). The detection rates are affected by the pathologist's expertise, the examination time and the amount of concentrated fluid that's available for analysis.
Molecular analyses for such markers as MAGE (8), telomerase (18,22) and hnRNP A2/B1 (23) have been studied for the early detection of lung cancer with using bronchial lavage samples. However, most molecular tests are too cumbersome to be utilized in the clinical laboratory. In this study, two cancer specific markers and an immediate RNA preservation system were used. Our detection method was regular RT-PCR technology, and so it can be easily adapted as a routine clinical laboratory test.
The results of this study showed that the positive rates of detecting the MAGE A1-6 or SSX4 expressions in lung cancer tissue were more than 90%. Although the number of cases of adenocarcinoma was very limited, the positive rates with using the SSX4 expression in lung cancer tissue were similar to those of a prior study (14). Thus, these molecular markers may be useful for lung cancer detection in clinical specimens.
With using the bronchial wash fluids from lung cancer patients, the combined use of detecting the MAGE and SSX4 expressions increased the detection rate for adenocarcinoma compared to using MAGE alone (57.1% vs. 42.9%, respectively). Moreover, using the SSX4 expression was found to be more specific than that with using the MAGE A1-6 expression for detecting benign lung disease. Thus, the additional test using SSX4 was beneficial without reducing the specificity for detecting lung cancer.
The sensitivity and specificity for the MAGE and SSX4 molecular testing in the bronchial wash fluid was 75.0% and 88.6%, respectively. Therefore, it would seem that MAGE and SSX4 testing may be useful as a clinical method for detecting lung cancer. When a cytological examination is added to this, then a sensitive and specific lung cancer detection test would be easily available in the clinical laboratory.
The lower detection rate for the cytology studies on specimens with an advanced T stage might be explained by a reduced ratio of cancerous cells to degenerated cells (18). However, with molecular testing, the detection rates were increased with the advancing T stage; this suggests that using the RT-PCR technique would reduce the chance of missing cancer cells in the fluid specimen. In studies that have compared cytology and PCR, the results have shown that PCR was more sensitive than cytology for cancer detection (18,24,25). Thus, the low sensitivity of cytological evaluation might lead to missing cancer cells, and this may be due to the presence of a small number of cancer cells mixed in with alveolar macrophages and other nonmalignant cells in the specimen. For the specimens that had negative cytology for peripheral cancer, MAGE or SSX4 testing could detect 67% of the cancers.
Low dose CT scanning has been shown to be useful in screening for early peripheral non-small cell lung cancers (20); needle aspiration may be able to differentiate between malignant and benign disease, but its role is less certain for lesions ≤1 cm (15). Thus, MAGE A1-6 and SSX4 RT-PCR may provide a novel and useful approach for the early detection of peripheral lung cancer in such cases.
CONCLUSIONSIn conclusion, determining the MAGE and SSX4 expressions in combination could detect 66.7% of the peripheral lung cancer missed by cytological examination. The specificity of this molecular testing was very high. MAGE and SSX4 RT-PCR may be a useful additional tool for detecting lung cancer in bronchial wash fluid.
NotesThis work was supported by grant No. RTI04-03-01 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE) and Seoul National University. References1. Park JW, Kwon TK, Kim IH, Sohn SS, Kim YS, Kim CI, et al. A new strategy for the diagnosis of MAGE-expressing cancers. J Immunol Methods. 2002;266:79–86. PMID: 12133624
2. Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora JP, De Smet C, et al. Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics. 1994;40:360–369. PMID: 7927540
3. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. PMID: 1840703
4. Jheon S, Hyun DS, Lee SC, Yoon GS, Jeon CH, Park JW, et al. Lung cancer detection by a RT-nested PCR using MAGE A1--6 common primers. Lung Cancer. 2004;43:29–37. PMID: 14698534
5. Bedrossian CW, Rybka DL. Bronchial brushing during fiberoptic bronchoscopy for the cytodiagnosis of lung cancer: comparison with sputum and bronchial washings. Acta Cytol. 1976;20:446–453. PMID: 1068615
6. Halling KC, Rickman OB, Kipp BR, Harwood AR, Doerr CH, Jett JR. A comparison of cytology and fluorescence in situ hybridization for the detection of lung cancer in bronchoscopic specimens. Chest. 2006;130:694–701. PMID: 16963665
7. Chan EC, Lam SY, Tsang KW, Lam B, Ho JC, Fu KH, et al. Aberrant promoter methylation in Chinese patients with non-small cell lung cancer: patterns in primary tumors and potential diagnostic application in bronchoalevolar lavage. Clin Cancer Res. 2002;8:3741–3746. PMID: 12473584
8. Mecklenburg I, Stratakis DF, Huber RM, Häussinger K, Morresi-Hauf A, Riethmüller G, et al. Detection of melanona antigen-A expression in sputum and bronchial lavage fluid of patients with lung cancer. Chest. 2004;125:164S–166S. PMID: 15136490
9. Xinarianos G, Scott FM, Liloglou T, Prime W, Turnbull L, Walshaw M, et al. Evaluation of telomerase activity in bronchial lavage as a potential diagnostic marker for malignant lung disease. Lung Cancer. 2000;28:37–42. PMID: 10704707
10. Fielding P, Turnbull L, Prime W, Walshaw M, Field JK. Heterogeneous nuclear ribonucleoprotein A2/B1 up-regulation in bronchial lavage specimens: a clinical marker of early lung cancer detection. Clin Cancer Res. 1999;5:4048–4052. PMID: 10632338
11. Gure AO, Wei IJ, Old LJ, Chen YT. The SSX gene family: characterization of 9 complete genes. Int J Cancer. 2002;101:448–453. PMID: 12216073
12. Chi SN, Cheung NK, Cheung IY. Expression of SSX-2 and SSX-4 genes in neuroblastoma. Int J Biol Markers. 2002;17:219–223. PMID: 12521124
13. Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85:713–720. PMID: 11531257
14. Tajima K, Obata Y, Tamaki H, Yoshida M, Chen YT, Scanlan MJ, et al. Expression of cancer/testis (CT) antigens in lung cancer. Lung Cancer. 2003;42:23–33. PMID: 14512184
15. Pasic A, Postmus PE, Sutedja TG. What is early lung cancer? A review of the literature. Lung Cancer. 2004;45:267–277. PMID: 15301867
16. Paik HC, Lee DY, Lee HK, Kim SJ, Lee KB. Chest wall implantation of carcinoma after fine needle aspiration biopsy. Yonsei Med J. 1994;35:349–354. PMID: 7975745
17. Voravud N, Shin DM, Dekmezian RH, Dimery I, Lee JS, Hong WK. Implantation metastasis of carcinoma after percutaneous fine-needle aspiration biopsy. Chest. 1992;102:313–315. PMID: 1623781
18. Yahata N, Ohyashiki K, Ohyashiki JH, Iwama H, Hayashi S, Ando K, et al. Telomerase activity in lung cancer cells obtained from bronchial washings. J Natl Cancer Inst. 1998;90:684–690. PMID: 9586665
19. Jones AM, Hanson IM, Armstrong GR, O'Driscoll BR. Value and accuracy of cytology in addition to histology in the diagnosis of lung cancer at flexible bronchoscopy. Respir Med. 2001;95:374–378. PMID: 11392578
20. Hirsch FR, Franklin WA, Gazdar AF, Bunn PA. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001;7:5–22. PMID: 11205917
21. Ahmed A, Ahmed S. Comparison of bronchoalveolar lavage cytology and transbronchial biopsy in the diagnosis of carcinoma of lung. J Ayub Med Coll Abbottabad. 2004;16:29–33. PMID: 15762059
22. Sen S, Reddy VG, Guleria R, Jain SK, Kapila K, Singh N. Telomerase - a potential molecular marker of lung and cervical cancer. Clin Chem Lab Med. 2002;40:994–1001. PMID: 12476937
23. Fielding P, Turnbull L, Prime W, Walshaw M, Field JK. Heterogeneous nuclear ribonucleoprotein A2/B1 up-regulation in bronchial lavage specimens: a clinical marker of early lung cancer detection. Clin Cancer Res. 1999;5:4048–4052. PMID: 10632338
24. Lobo TT, Feijo G, Carvalho SE, Costa PL, Chagas C, Xavier J, et al. A comparative evaluation of the Papanicolaou test for the diagnosis of trichomoniasis. Sex Transm Dis. 2003;30:694–699. PMID: 12972792
25. Chakravorty S, Sen MK, Tyagi JS. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357–4362. PMID: 16145077
Fig. 1Amplification of MAGE A1-6 (A), SSX4 (B) and GAPD (C) from ten (1~10) bronchial wash specimens of lung cancer patients. P=positive control, N=negative control. 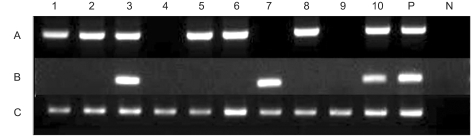
Fig. 2The positive detection rates of cytology, MAGE A1-6 and SSX4 with using bronchial wash fluids of lung cancer patients were evaluated by the T stage. *Statistically different detection rates (p<0.05) were observed at each T stage when cytology was compared with the other tests, †Cytology, MAGE and SSX4. 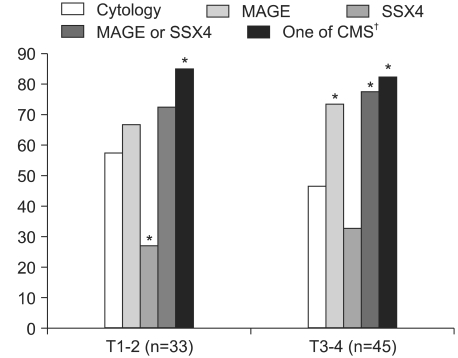
Fig. 3The positive detection rates of cytology, MAGE and SSX4 with using the bronchial wash fluids from lung cancer patients were analyzed by cancer locations. *Statistically different detection rates (p<0.05) compared with cytologic examination were observed according to the cancer location, †Cytology, MAGE and SSX4. 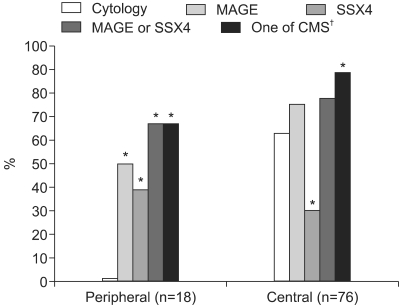
Table 1Positive expression rates of the MAGE A1-6 and SSX4 genes in the lung cancer tissues obtained by bronchoscopic biopsy 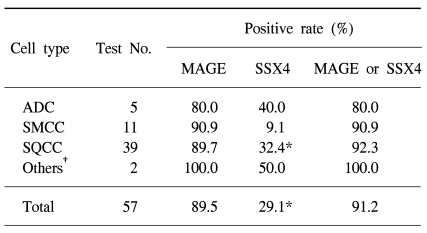
|
|
||||||||||||||||||||||||||||||||||||||