AbstractPurposeAlthough the use of xenograft models is increasing, few studies have compared the clinical features or outcomes of epithelial ovarian cancer (EOC) patients according to the tumorigenicity of engrafted specimens. The purpose of this study was to evaluate whether tumorigenicity was associated with the clinical features and outcomes of EOC patients.
Materials and MethodsEighty-eight EOC patients who underwent primary or interval debulking surgery from June 2014 to December 2015 were included. Fresh tumor specimens were implanted subcutaneously on each flank of immunodeficient mice. Patient characteristics, progression-free survival (PFS), and germline mutation spectra were compared according to tumorigenicity.
ResultsXenografts were established successfully from 49 of 88 specimens. Tumorigenicity was associated with lymphovascular invasion and there was a propensity to engraft successfully with high-grade tumors. Tumors from patientswho underwent non-optimal (residual disease ≥ 1 cm) primary orinterval debulking surgery had a significantly greater propensity to achieve tumorigenicity than those who received optimal surgery. In addition, patients whose tumors became engrafted seemed to have a shorter PFS and more frequent germline mutations than patients whose tumors failed to engraft. Tumorigenicity was a significant factor for predicting PFS with advanced International Federation of Gynecology and Obstetrics stage and high-grade cancers.
ConclusionsTumorigenicity in a xenograft model was a strong prognostic factor and was associated with more aggressive tumors in EOC patients. Xenograft models can be useful as a preclinical tool to predict prognosis and could be applied to further pharmacologic and genomic studies on personalized treatments.
IntroductionEpithelial ovarian cancer (EOC) remains a leading cause of gynecologic cancer-related mortality because it exhibits marked heterogeneity at the molecular, cellular, and clinical levels. This results in a high recurrence rate after standard primary treatment [1,2]. To deal with the heterogeneity, personalized medicine is now widely considered for intractable EOC, and its possible value is a reason for global research on this approach [3].
To precisely represent the biologic characteristics of an individual patient, previous studies have established disease models including two-dimensional cell lines and patient-derived xenograft models [4,5]. Compared with cancer cell lines, xenograft models can better characterize features of the original tumor, including tumor heterogeneity [6,7]. Therefore, xenograft models have become a widely used preclinical tool for developing treatment strategies in refractory EOC [2,8].
The rate of successful tumorigenicity of cancer specimens from EOC patients has been variable in previous studies. Furthermore, a considerable proportion of tumor samples failed to engraft successfully in xenograft models [9,10]. Successful engraftment may reflect aggressiveness of the primary tumor. Although the use of xenograft models has been increasing, few studies have compared the clinical features and outcomes of EOC patients according to tumorigenicity of the engrafted specimens [9-11]. Investigation of the relationship between engraftment tumorigenicity and EOC pathogenicity may provide better insight into the interpretation of findings of preclinical studies using xenograft models. Thus, the present study was designed to determine whether tumorigenicity correlates with the clinical features and outcomes of EOC patients.
Materials and Methods1. PatientsEighty-eight patients who were diagnosed with EOC from June 2014 to December 2015 were enrolled in this study. Included patients underwent one of the following two types of surgeries: (1) primary cytoreductive and (2) interval debulking following diagnostic laparoscopy.
2. Establishment of an ovarian cancer xenograft modelFresh tumor tissues from consenting patients with ovarian cancer were collected at the time of debulking (primary or interval) surgery. Samples were minced into small fragments with an operating scissors in RPMI 1640 medium (LM011-03, Welgene, Gyeongsan, Korea) containing a 1× antibioticantimycotic solution (15240-062, Gibco, Grand Island, NY).
Tumor cells were implanted subcutaneously on each flank of nude and NOG mice (S1 Video). Mice were anesthetized by injection of Zoletil/Rompun (7:3 ratio) and shaved on the rump where the surgery would occur. The site was disinfected with povidone iodine pads and 70% isopropyl alcohol swabs. A 2-mm long incision was made in the skin at the rump, and 100 μL of previously chopped tumor fragments were placed into each flank using a 10-gauge trocar. After implantation, the skin was sutured and the mice revived.
Tumor size was evaluated 2 to 3 times per week with a digital caliper (S2 Video). The tumor volume was calculated by the formula: 1/2 (width2 × length). Implants that reached a volume of 500 mm3 were considered tumorigenic.
The tumor from a founder mouse was expanded with a single passage into 5 to 10 mice to generate sufficient tumor volume for banking and future experiments. Harvested tissue was cryopreserved in 1 mL cryovials using 10% dimethyl sulfoxide for 72 hours in a −70°C freezer. After freezing, samples were transferred to liquid nitrogen tanks for a minimum of 24 hours before thawing in a 37°C water bath.
3. Clinicopathologic analysisPatient characteristics according to tumorigenicity of the engrafted tumor were compared. Patients were grouped according to the type of surgery: (1) primary cytoreductive including fertility-sparing and (2) diagnostic laparoscopy followed by interval debulking. All the patients included in the latter group received homogeneous regimen (i.e., paclitaxel and carboplatin) as neoadjuvant chemotherapy for 3 times, prior to interval debulking surgery. The following data were extracted from the medical records and compared according to the tumorigenicity: age, preoperative carbohydrate antigen 125 (CA-125) level, BRCA1/2 mutation, origin of the disease, histologic type, International Federation of Gynecology and Obstetrics (FIGO) stage, grade, lymphovascular invasion, lymph node metastasis, type of surgery, American Society of Anesthesiologists physical status, and residual disease.
4. Comparison of germline DNA mutation spectra with tumorigenicityTo investigate the germline mutational status of patients with and without tumorigenicity, we performed the next-generation sequencing assay using a 35-multigene test panel. We selected about ten patients from each group and determined the germline DNA mutations of 35 genes to compare the mutation spectra. Germline DNA was extracted from a peripheral blood sample of the participants using the QIAamp Blood DNA mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Intact DNA was quantified and adjusted to a concentration of 5 ng/μL using a Qubit 2.0 fluorometer and the Qubit dsDNA HS Assay Kit (Invitrogen, Waltham, MA). Pre-capture libraries were constructed according to the manufacturer’s sample preparation protocol. Each patient’s genomic DNA was fragmented with a median size of 300 bp. A customized targeted capture sequencing panel (OncoRisk, produced by Celemics, Seoul, Korea) was used that covered all coding sequences and intron-exon boundaries of coding exons from 35 cancer susceptibility genes including: BRCA1, BRCA2, PALB2, BARD1, BRIP1, RAD51C, RAD51D, RAD50, NBN, MRE11A, ATM, CHEK2, TP53, PTEN, APC, BLM, BMPR1A, CDH1, CDK4, CDKN2A, EPCAM, MEN1, MLH1, MSH2, MSH6, MUTYH, PMS2, POLE, PRSS1, RET, SLX4, SMAD4, STK11, VLH, and WT1. The DNA fragments were end-repaired, phosphorylated, and adenylated on the 3′ ends. Index adaptors were ligated to the repaired ends, DNA fragments were amplified, and fragments of 200 to 500 bp were isolated. Pooled libraries were sequenced on a MiSeq sequencer (Illumina, San Diego, CA) using the MiSeq Reagent Kit v2 (300 cycles).
5. Statistical analysesSPSS ver. 23.0 for Windows (IBM Corp., Armonk, NY) was used to analyze data. The chi-square or Fisher exact tests were used to compare engraftment status with patient characteristics. Survival outcomes were determined through a Kaplan-Meier survival analysis. Univariate and multivariate analyses of the effects of various prognostic factors on survival were performed using the Cox proportional hazards model. Multivariate analysis was performed with variables that were considered significant in the univariate analysis. p-values of less than 0.05 were considered statistically significant.
6. Ethical statementThis study was reviewed and approved by our Institutional Review Board (IRB No. 4-2013-0526), and was performed in accordance with the ethical standards described in the Declaration of Helsinki. The requirement to obtain a written informed consent was waived by the Institutional Review Board of the Yonsei University College of Medicine because our study was retrospective research, and this research presented no more than minimal risk of harm to subjects. All animal experiments were performed according to protocols approved by the appropriate institutional review board and conducted in accordance with the National Institutes of Health Laboratory Animal Research Guide for the Care and Use ofLaboratory Animals.
Results1. Patient characteristics and tumorigenicityOut of 88 tumor specimens, 49 (53.4%) were engrafted successfully. Patient characteristics and comparisons according to tumorigenicity are shown in Table 1. The patients had a median age of 52 (range, 27 to 82), and 83% carried advanced FIGO stage cancer. The subjects underwent one of two types of treatment including primary debulking surgery (72.7%) and interval debulking surgery (27.3%). Seventy-six patients received optimal surgery that resulted in a residual tumor size of less than 1 cm.
Tumorigenicity was correlated with a high-grade tumor, lymphovascular invasion, and residual disease after debulking surgery. There were no significant differences between the two debulking surgery groups according to tumorigenicity and age, preoperative CA-125 levels, lymph node metastasis, histology, and stage.
2. Clinical outcomes and tumor engraftmentMedian follow-up period was 21 months, ranging 1 to 100 months. Univariate and multivariate Cox regression analyses of progression-free survival (PFS) in all patients are shown in Table 2. Multivariate analysis of PFS identified advanced FIGO stage, high-grade tumor, and tumorigenicity as significant factors (p=0.270, p=0.026, and p=0.021, respectively). The hazard ratio of tumorigenicity for PFS was 2.196 (95% confidence interval, 1.123 to 4.292).
A Kaplan-Meier survival analysis indicated an apparently unfavorable PFS in the tumorigenicity-positive compared to the tumorigenicity-negative group, although this difference was not significant (p=0.097) (Fig. 1A). In the subgroup analysis, positive tumorigenicity correlated significantly with a poor PFS when compared with negative tumorigenicity among patients who underwent primary debulking surgery (p=0.044) (Fig. 1B). Among patients who underwent interval debulking surgery, there was no difference in PFS regarding tumorigenicity (Fig. 1B).
3. Germline DNA mutation spectra versus tumorigenicityThe spectra of germline mutations are presented in Fig. 2. Six of 11 patients whose tumor samples engrafted successfully showed six pathogenic mutations. These included four nonsense mutations in BRCA1 and BRCA2, and two splicing mutations in BRCA1 and MUTYH. Three pathogenic mutations were evident in three of eight patients whose tumor samples failed to engraft. These three mutations were all frame shifts in BRCA1, BRCA2, and RAD51D.
DiscussionWe compared the clinical features and outcomes of patients with EOC in relation to successful tumorigenicity in a xenograft model. Similar to previous studies [9,10], xenografts were established successfully with 53.4% of the primary tumors. Tumorigenicity of the grafted tumors correlated with lymphovascular invasion and failure to achieve optimal surgery. Successful tumor engraftment in the xenograft model also appeared to be associated with high-grade tumors. We observed an apparently unfavorable PFS in the tumorigenicity-positive compared to the tumorigenicity-negative group, although this difference was not significant. Specifically, in patients who underwent primary debulking surgery and whose tumors failed to engraft, there was significant improvement in PFS. In addition, tumorigenicity was an independent predictor of PFS. These results suggest that EOCs with more aggressive features are better able to engraft than less aggressive cancers.
In our study, tumorigenicity was significantly correlated with PFS in chemotherapy-naïve cancer. This finding implied that engraftment failure of chemotherapy-naïve tumors reflected low aggressiveness of the primary tumor. However, after cytotoxic chemotherapy had affected the cancer tissue, failure of tumor engraftment did not appear to represent low aggressiveness of the primary tumor because there was poor PFS regardless of tumorigenicity. This observation suggested that, after neoadjuvant chemotherapy, even aggressive malignant cells could fail to engraft in the xenograft model. Considering the high chemosensitivity of EOC, neoadjuvant chemotherapy would greatly reduce the tumor bulk. This may result in low cellularity of the tumor sample obtained during debulking surgery [12]. In other words, it appears that the low cellularity of the cancer specimen, not the low aggressiveness of the primary tumor, resulted in the failure of engraftment.
Our results demonstrated that tumorigenicity could be used as a strong predictive factor for aggressiveness of the primary tumor regarding lymphovascular invasion, high-grade, and failure in the achievement of optimal surgery. In particular, because optimal debulking surgery is a major factor that impacts survival in advanced ovarian cancer [13], tumor engraftment can act as a useful indicator of survival outcome. In our study, there was a marginally significant improvement in PFS in the negative tumorigenicity group, and significant improvement in the negative tumorigenicity group treated with primary debulking surgery, indicating the possible role of tumorigenicity as a predictive factor.
A few previous studies have evaluated the relationship between successful tumorigenicity in a xenograft model and clinical outcomes of EOC patients [9,10]. In one study using 45 xenografts, patients whose tumors were successfully engrafted in mice had significantly inferior overall survival when compared with those whose tumors failed to engraft (p=0.040) [10]. In another study, 168 xenografts were analyzed. The authors reported that patients whose tumors successfully engrafted in mice had inferior overall survival (p=0.059) relative to patients whose tumors did not successfully engraft [9]. Comparable studies have been performed for colon cancer, non-small cell lung cancer, breast cancer, and uveal melanoma [3,11,14-16]. A study for non-small cell lung cancer showed that tumorigenicity correlated with the presence of KRAS mutations, poor differentiation, and larger tumor size. In addition, tumorigenicity was an independent predictor of shorter disease-free survival [14]. In contrast, another non-small cell lung cancer study reported that tumorigenicity did not correlate with clinical outcomes [15]. The previous studies on the correlation of engraftment in xenograft models and clinical outcomes, however, did not perform a subgroup analysis for investigating the possible influence of cytotoxic chemotherapy on tumorigenicity.
Numerous genomic mutations have been identified in patients with EOC, and high levels of oncogene mutation can accelerate tumor engraftment [17-19]. In our study, we performed the next-generation sequencing assay and compared the spectra of germline mutations relative to tumorigenicity in the xenograft model. We detected several mutations of EOC-related genes. Mutation of BRCA1 and BRCA2 were detected most frequently, especially among patients with successful tumor engraftment. According to previous reports, xenograft models can accurately represent the genetic diversity of the primary tumor and better predict clinical tumor response to new therapeutics, such as molecularly targeted therapy [10,11]. Further studies that analyze somatic mutations of our engrafted tumors could verify the utility of the xenograft model for drug sensitivity testing followed by molecularly targeted therapy, especially those with disease refractory to conventional treatments [8,20].
Well-characterized xenograft models are useful in developing novel targeted therapies [21,22]. In the current study, xenografts were stably established, and we observed that successful tumorigenicity of patient-derived EOC specimens correlated with more aggressive cancer features and worse oncologic outcomes. Therefore, xenograft models may play a role as effective preclinical tools to evaluate cancer progression and to determine treatment strategies. These models can be applied to further pharmacologic and genomic research on personalized therapies. Our results provide evidence that xenograft models are relevant to EOC patients with a refractable course or high risk of relapse. We expect that personalized treatments with the preclinical use of xenograft models will improve survival rates for EOC patients.
In conclusion, our study showed that the successful tumorigenicity of EOC specimens in a xenograft model was associated with more aggressive disease and worse prognosis. In particular, failure of chemotherapy-naïve tumor engraftment indicated low aggressiveness of the primary tumor that could result in a favorable prognosis. As a preclinical tool, xenograft models may be helpful to predict disease progression for personalized treatment and, finally, to improve clinical outcomes of EOC patients. Further pharmacologic and genomic studies on personalized treatments using the xenograft model are expected to provide novel treatments for retractable EOC patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
AcknowledgmentsThis work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2014R1A1A1A05002-926; 2015R1A2A2A01008162; 2015R1C1A2A01053516), a faculty research grant of Yonsei University College of Medicine (6-2016-0088), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (2016-31-0454, HI13-C2162), funded by the Ministry of Health & Welfare, Republic of Korea. Partial funding was provided by the Korea Gynecologic Cancer Bank through the Bio & Medical Technology Development Program of the MSIP, Korea.
Fig. 1.Comparison of progression-free survival in patients relative to the tumorigenicity of engrafted tumors in a xenograft model. (A) Comparison in 88 patients. (B) Subgroup analysis by type of debulking surgery: primary and interval debulking. PDS, primary debulking surgery; IDS, interval debulking surgery. 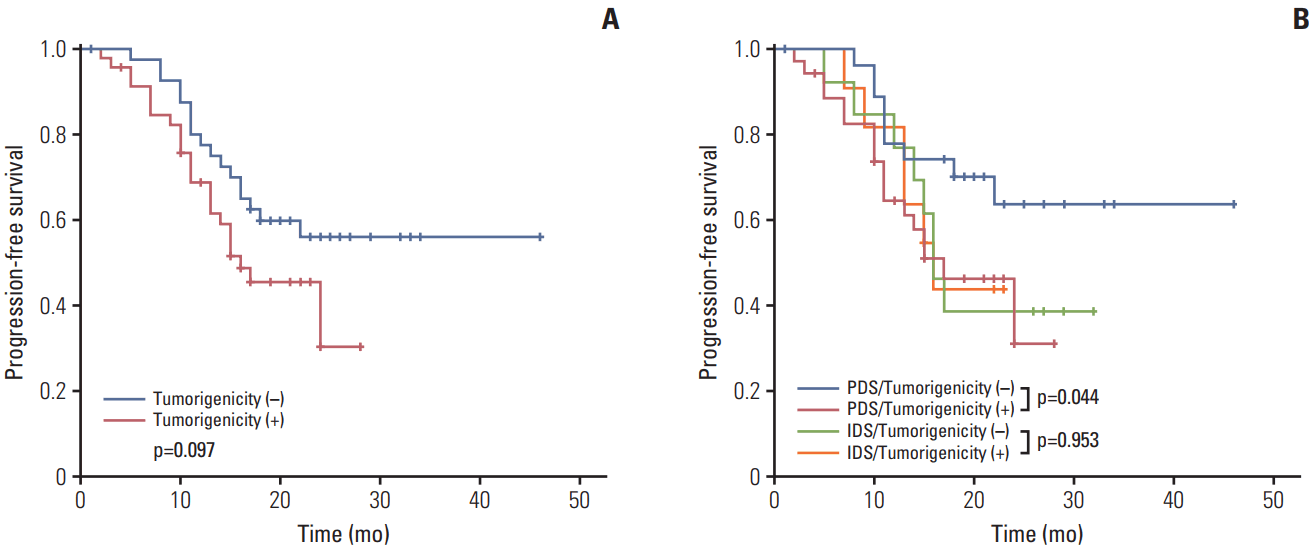
Fig. 2.Comparison of germline mutation spectra relative to tumorigenicity using a 35-multigene panel next-generation sequencing assay. VOUS, variants of unknown significance. 
Table 1.Patient characteristics in relation to the tumorigenicity of engrafted tumors in a xenograft model Table 2.Univariate and multivariate analyses of various factors for progression-free survival References2. Erriquez J, Olivero M, Mittica G, Scalzo MS, Vaira M, De Simone M, et al. Xenopatients show the need for precision medicine approach to chemotherapy in ovarian cancer. Oncotarget. 2016;7:26181–91.
3. Nemati F, Sastre-Garau X, Laurent C, Couturier J, Mariani P, Desjardins L, et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res. 2010;16:2352–62.
4. Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–80.
5. Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009;85:217–21.
6. Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13:3989–98.
7. Puig I, Chicote I, Tenbaum SP, Arques O, Herance JR, Gispert JD, et al. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res. 2013;19:6787–801.
8. Dobbin ZC, Katre AA, Steg AD, Erickson BK, Shah MM, Alvarez RD, et al. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget. 2014;5:8750–64.
9. Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014;20:1288–97.
10. Heo EJ, Cho YJ, Cho WC, Hong JE, Jeon HK, Oh DY, et al. Patient-derived xenograft models of epithelial ovarian cancer for preclinical studies. Cancer Res Treat. 2017;49:915–26.
11. Oh BY, Lee WY, Jung S, Hong HK, Nam DH, Park YA, et al. Correlation between tumor engraftment in patient-derived xenograft models and clinical outcomes in colorectal cancer patients. Oncotarget. 2015;6:16059–68.
12. Medina-Franco H, Cortes-Gonzalez R, Lambreton-Hinojosa F, Fimbres-Morales A, Vargas-Siordia JC. Neoadjuvant chemotherapy increases R0 cytoreduction rate but does not improve final outcome in advanced epithelial ovarian cancer. Ann Surg Oncol. 2017;24:1330–5.
13. Ataseven B, Grimm C, Harter P, Heitz F, Traut A, Prader S, et al. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol Oncol. 2016;140:215–20.
14. John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011;17:134–41.
15. Anderson TM, Hess SD, Egilmez NK, Nwogu CE, Lenox JM, Bankert RB. Comparison of human lung cancer/SCID mouse tumor xenografts and cell culture growth with patient clinical outcomes. J Cancer Res Clin Oncol. 2003;129:565–8.
16. DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–20.
17. Bobbs AS, Cole JM, Cowden Dahl KD. Emerging and evolving ovarian cancer animal models. Cancer Growth Metastasis. 2015;8(Suppl 1):29–36.
18. Topp MD, Hartley L, Cook M, Heong V, Boehm E, McShane L, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol Oncol. 2014;8:656–68.
19. Ricci F, Bizzaro F, Cesca M, Guffanti F, Ganzinelli M, Decio A, et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014;74:6980–90.
20. Li H, Zhu Y, Tang X, Li J, Li Y, Zhong Z, et al. Integrated analysis of transcriptome in cancer patient-derived xenografts. PLoS One. 2015;10:e0124780
|
|
|||||||||||||||||||||||||||||||||||||||||||||