AbstractPurpose Fecal tests remain a mainstay of population-based colorectal cancer (CRC) screening programs worldwide. However, data on interval CRC (iCRC) arising after follow-up colonoscopy of a positive fecal test are scarce. We conducted a nationwide population-based study to reveal the risk and characteristics of iCRC in this setting.
Materials and Methods We searched the National Cancer Screening Program for CRC database in Korea (2005-2010). Incidence of iCRC within the program was estimated, then Cox proportional-hazards regression analysis was performed to determine the independent predictors of iCRC. The clinical characteristics of iCRC were compared with screen-detected CRC (sCRC).
Results We identified 280 iCRC among 150,660 negative colonoscopies as a follow-up exam to a positive fecal immunochemical test (FIT), and 2,427 sCRC. The overall incidence of iCRC was 0.49/1,000 person-years (95% confidence interval [CI], 0.48 to 0.51). iCRC was more likely to occur in men (adjusted hazard ratio [aHR], 1.79; 95% CI, 1.39 to 2.30) and elderly patients (aHR, 1.77; 95% CI, 1.38 to 2.28 in 65-74 years; aHR, 3.13, 95% CI, 2.13 to 4.60 in ≥ 75 years). The National Quality Improvement Program for colonoscopy reduced a short-term risk of iCRC (aHR, 0.48; 95% CI, 0.27 to 0.87). Compared with sCRC, iCRC was more likely to occur in the proximal colon, be diagnosed at the localized stage, and have a lower CRC mortality (32.7 vs. 17.4%, 56.8 vs. 34.1%, and 12.5 vs. 17.7%, respectively; all p < 0.05).
IntroductionColorectal cancer (CRC) is one of the most common malignancies, and it is the leading cause of cancer-related deaths worldwide [1]. Colonoscopy screening is recently being more accepted as a primary modality of CRC screening, but fecal occult blood tests (FOBT) still remain a mainstay of population-based CRC screening programs in many countries [2]. Irrespective of the type of fecal tests used, however, a final diagnosis of CRC is made through follow-up colonoscopy. Therefore, the success of an organized CRC screening program based on fecal tests is critically associated with the quality of colonoscopy [3].
In recent years, interval CRC (iCRC) developed after negative colonoscopy has emerged as a major issue related to the quality of colonoscopy [4,5]. As iCRC theoretically has a deleterious impact on screening outcomes, the rate of iCRC is now accepted as a key performance indicator of organized CRC screening programs [3]. Although previous studies have reported the epidemiology, risk factors, and possible etiologies of iCRC, several key questions remain unsolved [6-12]. Among these, data regarding iCRC arising after diagnostic colonoscopy of a positive primary screening test are limited. Given that a follow-up examination of a positive fecal test is a common procedural indication for colonoscopy but has a high yield of CRC diagnosis, better characterization of iCRC in this setting would have important clinical impacts on policymaking for CRC screening programs as well as daily clinical practice.
In Korea, the incidence of CRC has dramatically increased over the past few years. The age-standardized incidence rates of CRC per 1,000 persons in men and women are estimated to be 50.3 and 27.7 cases respectively in 2012 [13]. The National Cancer Screening Program (NCSP) for CRC was implemented in 2004, and the program offers an annual testing with a single fecal immunochemical test (FIT) to all Korean men and women over 50 years of age, and follow-up examination with either colonoscopy (with biopsy if indicated) or double contrast barium enema for subjects with a positive FIT result [14]. In 2008, the Ministry of Health and Welfare launched the National Quality Improvement Program (NQIP) for colonoscopy, which aimed to ensure highquality colonoscopy for the target population of the NCSP for CRC.
In this context, we aimed to investigate the risks and characteristics of iCRC arising after negative follow-up colonoscopy of a positive FIT within a nationwide populationbased CRC screening program. Further, we examined the potential impact of the NQIP for colonoscopy on risk reduction of iCRC.
Materials and Methods1. Study population and CRC screening programData were obtained from the NCSP for CRC database, which contains information on the Medical Aid recipients and National Health Insurance Service (NHIS) beneficiaries invited to participate in the NCSP [15,16]. The NCSP offers an annual FIT to men and women over 50 years of age, and follow-up examination with either colonoscopy or double contrast barium enema for subjects with a positive FIT result.
The study cohort was comprised of cancer free subjects who received diagnostic colonoscopy (designated as index colonoscopy) after a positive FIT result at any round of the screening program, between January 2005 and December 2010. The baseline population was comprised of 15,704,684 Korean men and women over 50 years of age who were invited to undergo screening via the NCSP for CRC during the study period. In total, 6,337,086 participants underwent FIT, 534,661 of whom had a positive FIT result (8.0%). Of these, 153,678 subjects with a positive FIT result who underwent a diagnostic colonoscopy were included in this study (Fig. 1). Written informed consent was received from participants for the collection of their screening results. This study was approved by the Institutional Review Board of the National Cancer Center, Korea (NCCNCS08129).
2. Case definitionWithin the NCSP for CRC, the results of diagnostic colonoscopy with biopsy were judged as one of the following pre-defined statuses: “normal,” “colon polyp,” “suspected CRC,” “CRC,” or “other diagnoses.” We defined a positive colonoscopy result as “suspected CRC” or “CRC,” and defined a negative colonoscopy result as “normal,” “polyp,” or “other diagnoses.” Using resident registration numbers, all subjects were traced longitudinally by linkage to the death certificate database, and to the Korea Central Cancer Registry (KCCR), which covers over 97% of all newly-diagnosed malignancies in Korea, in order to identify subsequently developed cancers from January 2005 to December 2011 [17]. Then, we defined iCRC as CRC diagnosed within 6-60 months after index colonoscopy with a negative result, and screen-detected cancer (sCRC) as a CRC diagnosed within 6 months of the index colonoscopy with a positive result. Our definition of iCRC followed that of a recent population-based study [8]. All CRC diagnoses were made on the basis of pathologic confirmation from the KCCR data. We monitored patients for development of newly diagnosed CRC or patient death until 60 months after index colonoscopy.
For subgroup analysis, we categorized iCRC as early (CRC diagnosed within 6-36 months after index colonoscopy) or late (those diagnosed within 37-60 months after index colonoscopy) iCRC according to time since index colonoscopy. We also categorized iCRC as proximal (CRC occurring proximal to the splenic flexure: cecum, ascending colon, hepatic flexure, and transverse colon) or distal (those occurring in and distal to the splenic flexure: splenic flexure, descending colon, sigmoid colon, recto-sigmoid junction, and rectum) iCRC, according to tumor location.
3. Data extractionThe demographic variables used in this study were gender, age at index colonoscopy, hospital setting of colonoscopy procedure (primary clinic, community hospital, or general hospital), and calendar year of index colonoscopy. We also extracted the tumor profiles of diagnosed CRC from the KCCR, including date of CRC diagnosis and biological characteristics of the CRC (location, histology, and stage). The International Classification of Diseases for Oncology, Third edition (ICD-O-3) was used for topographic and histologic classification of all incidental CRCs [18]. We used the Surveillance, Epidemiology, and End Results Summary Staging 2000, in which CRCs are classified as localized, regional, distant, or unknown [19]. Data regarding all cause and cancer specific death were ascertained from the death certificates.
4. Statistical analysisTo estimate the risk of iCRC within the NCSP, we calculated the prevalence of iCRC as follows: the proportions of iCRC among all negative colonoscopies, and among all diagnosed CRCs (number of patients with iCRC divided by the total number of patients diagnosed with CRC). To calculate the incidence of iCRC, the number of patients with iCRC was divided by the sum of person-years follow-up after negative colonoscopy. The incidence of iCRC was presented as the estimated number of cases per 1,000 person-years followup with 95% confidence interval (CI).
Then we used a multivariate Cox proportional-hazards regression model to explore the association between several potential predictors and the risk of developing iCRC. Data in the regression analyses were presented as adjusted hazard ratios (HRs), with a 95% CI after adjustments for other explanatory variables. Additionally, we evaluated the effect of the NQIP for colonoscopy on risk reduction of iCRC. As described earlier, the NQIP for colonoscopy was launched in 2008. Thus, maximum follow-up period after introduction of the NQIP was 24 months. For comparability of iCRC rate before and after introduction of the NQIP, therefore, we included only iCRC arising within 6 and 24 months after index colonoscopy between 2005 and 2009 for this analysis.
To characterize iCRC, we compared patient demographics and tumor characteristics of patients with iCRC with those of patients with sCRC. Subgroup analyses in patients with iCRC were performed according to time since index colonoscopy (early vs. late) and tumor location (proximal vs. distal). Pearson chi-square or Fisher exact test was used to compare categorical variables, as appropriate. All statistical analyses were conducted using SAS ver. 9.3 (SAS institute Inc., Cary, NC). p-values of < 0.05 were considered statistically significant.
ResultsBetween 2005 and 2010, a total of 153,678 subjects with a positive FIT result underwent complete colonoscopy, and 150,660 of whom had a negative result in colonoscopy test. Of these, a total of 280 iCRC were identified. The prevalence of iCRC was 0.2% (280/150,660) among all negative colonoscopies and 10.3% (280/2,707) among all diagnosed CRC. In patients with iCRC, 67.1% were men, and 51.1% were 50-64 years old at index colonoscopy (Table 1).
1. Incidence and independent predictors of iCRCThe incidence of iCRC per 1,000 person-years follow-up in subjects with negative colonoscopy was estimated as 0.49 (95% CI, 0.48 to 0.51), 0.62 (95% CI, 0.60 to 0.65), and 0.35 (95% CI, 0.32 to 0.37) in overall, men, and women, respectively (Table 2). A multivariate Cox proportional-hazards regression model showed that male and older age at index colonoscopy were significantly associated with increased risk of iCRC. Adjusted HRs were estimated as 1.79 (95% CI, 1.39 to 2.30) in men, and 1.77 (95% CI, 1.38 to 2.28) and 3.13 (95% CI, 2.13 to 4.60) in 65-74 and ≥ 75 years of age, respectively. Hospital setting of colonoscopy procedure was not associated with the risk of iCRC. The calendar year of the index colonoscopy was also associated with risk of iCRC. The incidence of iCRC have significantly decreased since 2008. Fig. 2 depicts the time trend of the incidence of iCRC since index colonoscopy. To compare incidence rate of iCRC before and after the implementation of the NQIP for colonoscopy, we identified 123 patients with iCRC arising within 6-24 months of index colonoscopies that were performed between 2005 and 2009 (S1 Table). During 24 moths of follow-up, a statistically significant risk reduction of iCRC incidence was not observed in the first year (2008: adjusted HR, 0.63; 95% CI, 0.36 to 1.16). However, the risk of iCRC was significantly reduced in the second year of the program (2009: adjusted HR, 0.48; 95% CI, 0.27 to 0.87).
2. Interval versus screen-detected CRCWe compared 280 patients with iCRC and 2,427 patients with sCRC (Table 3). There were no significant differences in demographics between the two groups, except for the hospital setting of colonoscopy procedure (p=0.03). A larger proportion of iCRCs were diagnosed at the localized stage, located in the proximal colon, and had a lower mortality due to CRC, compared with sCRC (56.8% vs. 34.1%, 32.7% vs. 17.4%, 12.5% vs. 17.7%, respectively; all p < 0.05). More specifically, iCRC was more likely to occur in the cecum, ascending colon, and hepatic flexure compared with sCRC (Table 4). In contrast, sCRC was more likely to occur in the sigmoid colon, recto-sigmoid junction, and rectum compared with iCRC. However, the most common sites of iCRC were identical to those of sCRC: the rectum, sigmoid colon, and ascending colon, in order of frequency.
3. Subgroup analysis of iCRCAmong all iCRCs, 77.1% (216/280) occurred within 6-36 months after the index colonoscopy, with an early-to-late iCRC ratio of 3.38; and 61.9% occurred in the distal colon, mostly from the rectum through the sigmoid colon (57.6%) (Table 5). More patients with proximal iCRC were elderly at index colonoscopy (16.5% vs. 8.7%), and their iCRC was diagnosed at an advanced stage (regional or distant), compared with patients with distal iCRC (44% vs. 29%).
DiscussionIn this nationwide population-based study, we characterized iCRC after diagnostic colonoscopy as a follow-up exam of a primary FIT screening. We reported the prevalence of iCRCs as 0.2% among all negative colonoscopies and as 10.3% among all diagnosed CRCs within our program. The latter rate is a little bit higher than those of recent population-based studies from Canada and the United States (7.2%-9.0% among total CRCs) [9-11]. The current data, however, may seem reasonable, considering that previous studies defined iCRC as CRC diagnosed within 6-36 months after colonoscopy [9-11]. When confined to early iCRC developed within 6-36 months after index colonoscopy, the prevalent rate of iCRC in the current study is 7.9% among total CRCs (216/2,707). It has been suggested that the time cut-off of 36 months in the definition of iCRC may miss the detection of slowly growing precursor lesions, underestimating the real burden of iCRC [7,8,20,21]. Moreover, previous studies based on administrative or nationwide data are commonly hampered by the variability of colonoscopy indication, and often by sampling bias or small numbers of iCRC [6-12]. In this regard, a unique strength of this study was that we could capture iCRCs arising after colonoscopy as a follow-up exam of a positive FIT. Further, this large cohort was derived from the NCSP for CRC targeting the entire Korean population over 50 years of age. We used confident data regarding tumor profiles by linkage to the KCCR, which covers over 97% of all newly diagnosed malignancies in Korea. We believe that our efforts might contribute to minimize the risk of over- or under-estimation of the burden of iCRC, and that our data could be used as the reference standard for evaluating the screening outcomes of organized population-based CRC screening programs.
In this study, the incidence of iCRC was remarkably higher in men and elderly patients than in their counterparts (Table 2). The results clearly indicate that physicians should give added attention to their colonoscopy practice as a follow-up examination of positive fecal tests, especially in men and elderly patients. These associations can be partly explained by the background CRC incidence [13,14]. In Korea, the incidence rate of CRC increased gradually with age, and was the highest in age 80-85 years in both male and female. Also, the CRC incidence was almost two times higher in male than in female. Therefore, the reported increase in CRC incidence with increasing age in male would likely account for the higher iCRC rates noted in this study. In addition, our results might be partly attributable to the procedural indication of colonoscopy (diagnostic confirmatory colonoscopy after a positive fecal test). Two previous studies support our findings [22,23]. A German population-based study reported that follow-up of a positive fecal test was significantly associated with the occurrence of iCRC after colonoscopy in men (odds ratio, 5.49; 95% CI, 2.10 to 14.35) [22]. Age was an independent risk factor for iCRC after negative colonoscopy in the Taiwanese Nationwide CRC screening program with fecal tests (adjusted relative risk, 1.82; 95% CI, 1.19 to 2.78) [23]. In our study, the hospital setting where index colonoscopies were performed was not associated with the risk of iCRC. This result was contrary to our expectation that iCRCs would be less likely to occur in a large-volume center, although prior studies have shown inconsistent results related to this issue [8,10]. Physician specialty could be a potential answer to our finding [8,9-11], but our database did not contain the details of physician specialty. This was a potential limitation of our study.
The current study also demonstrated that iCRCs were featured by the proximal colon in location, and an early (localized) stage at diagnosis, compared with sCRCs (Table 3). A recent meta-analysis evaluating 12 studies reported that iCRCs were less likely to be diagnosed at an advanced stage compared with detected CRCs, and proximal iCRCs are 2.4-times more likely to develop than distal iCRCs [6]. It might be explained by the fact that proximally located colorectal neoplasms are more often small and flat than distal cancers, thereby contributing to the limited effectiveness of colonoscopy in the proximal colon [24]. Thus, risk reduction after negative colonoscopy was less pronounced for proximal cancer, especially cancer in the caecum and ascending colon [25].
In the current study, the most striking difference with previous studies is the anatomical site distribution of iCRCs (Table 4). The proportion of distal iCRC in this study (61.9%) is higher than those of previous population-based Caucasian studies, reporting rates of 29.9% to 46.3% among all iCRCs [9-11]. The anatomical site distribution of iCRCs is consistent with incidence of CRC by subsite in Korea: the rectum was the most common CRC site among Korean, followed by the distal colon and the proximal colon [26]. Thus, possible explanation for our contradictory finding with previous studies may be the result of ethnic or racial differences in incidence of CRC by subsite. The study conducted in the United States reported that that the rectum was the most common subsite for male Asians and Pacific Islanders living in the United States (35%), whereas the proximal colon was the most common site for Whites and Blacks among both men and women [27,28].
Meanwhile, a similar anatomical site distribution between iCRCs and sCRCs also suggests that the iCRCs detected in our study might be missed lesions that could possibly be preventable by a primary fecal test and confirmatory colonoscopy. It is well-known that most FOBT-detectable cancers in CRC screening program are likely to be located in the left colon and rectum [29]. Given the inherent anatomical characteristics of the distal colon and rectum, where acute bends and/or redundancy of the bowel frequently make blind spots for colonoscopy, more attention should be paid during colonoscope withdrawal.
The NQIP for colonoscopy in Korea was aimed to increase the quality of follow-up colonoscopy after a primary screening test. Through this program, all participating endoscopists were obliged to earn a given number of credits in educational programs. Two independent gastroenterologists from academic hospitals who were commissioned for the program, also performed site audit. All assessments of colonoscopy quality were conducted by using the Endoscopy Quality Rating Scale (EQRS) for colonoscopy [30]. We expected that such a specific, integrated intervention would reduce the burden of iCRC. The current study demonstrated that the short-term risk of iCRC arising within 6-24 months after index colonoscopies significantly decreased in 2009, the second year of the program (adjusted HR, 0.48; 95% CI, 0.27 to 0.87). Further prospective trials are needed to verify the long-term impact of quality control programs in colonoscopy on the outcomes of organized screening programs.
Our study has several limitations. First, our definition of negative colonoscopy included a category of polyp, rather than complete negative colonoscopy without any adenomas or other neoplastic lesions. Therefore, we might not completely exclude a potential bias related to case identification with iCRC. As stated earlier, however, the final judgment of colonoscopy results within the NCSP was based on the biopsy results. All diagnoses of CRC were made on the basis of pathologic confirmation from the KCCR data. Secondly, our estimation of the burden of iCRC might be underestimated, as the patients who underwent their colonoscopies in 2009 and 2010 had not fulfilled our planned follow-up interval (60 months after colonoscopy) at the time of analysis. Given that our study was one of the largest nationwide studies, however, we believe that our results adequately reflected the real burden of iCRC.
In conclusion, iCRCs after diagnostic colonoscopy occur at a substantial frequency in a mass CRC screening based on FIT, especially in men and elderly patients. The distal colon predominance of iCRCs and their similar site distribution with sCRCs strongly suggest missed lesions as the most possible explanation for occurrences of iCRCs in this setting. Although this study provided significant results in understanding the circumstances around iCRC after colonoscopy in Korea, there is still work to be done to prevent. The most important ways to reducing iCRC will involve both refinements in colonoscopy as an exam, and improvements in the way that it is delivered. Continuous quality improvement program for confirmatory colonoscopy is required as an integral part of organized screening programs for CRC.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
AcknowledgmentsThis study was supported by a Grant-in-Aid for Cancer Research and Control from the National Cancer Center, Korea (Grant number: 1610401).
Fig. 1.Flow chart of the study population and case identification. NCSP, National Cancer Screening Program; CRC, colorectal cancer; FIT, fecal immunochemical test. 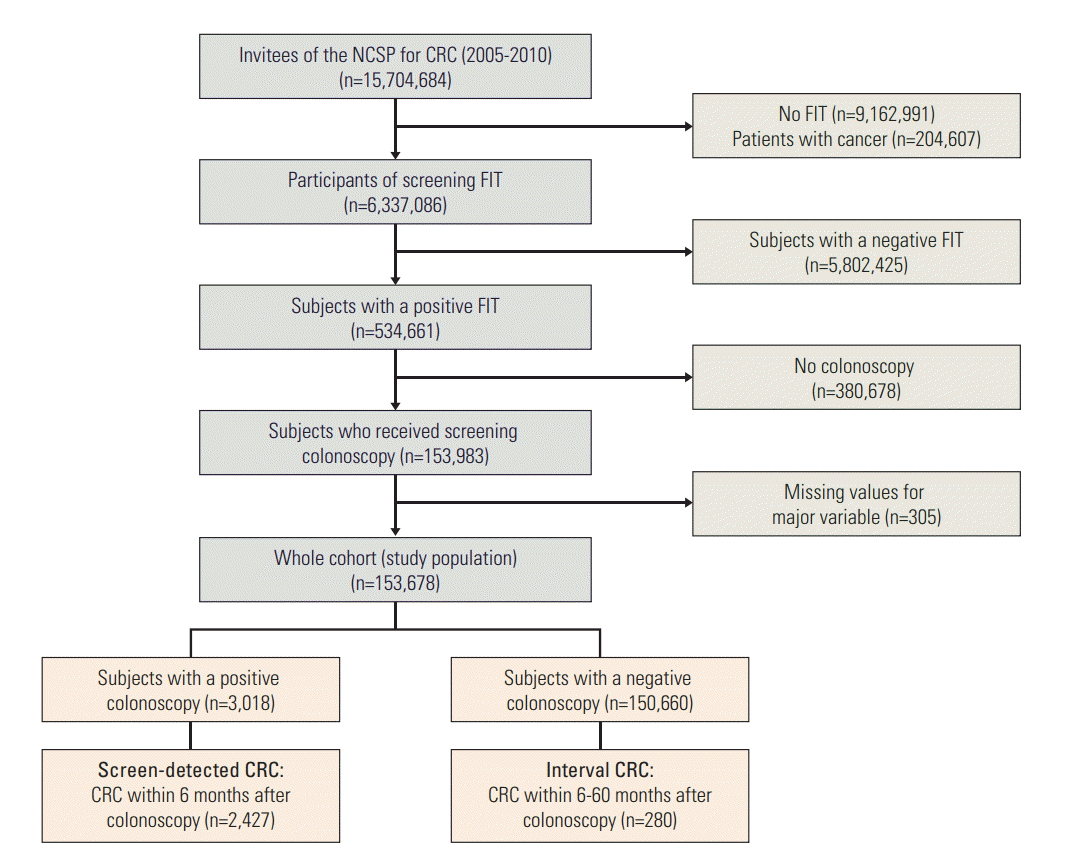
Table 1.Demographics of study cohort
Table 2.Incidence rates of iCRCs
Table 3.Basic characteristics of interval cancers compared with screen-detected cancers
Table 4.The subsite distribution of interval cancers and screen-detected cancers Table 5.The subgroup analysis of interval colorectal cancers
References1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
2. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49.
3. Moss S, Ancelle-Park R, Brenner H; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition: evaluation and interpretation of screening outcomes. Endoscopy. 2012;44 Suppl 3:SE49–64.
4. Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306.
5. Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803.
6. Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375–89.
7. Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949–56.
8. le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–63.
9. Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044–52.
10. Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72.
11. Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–96.
12. Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010;105:663–73.
13. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–41.
14. Shim JI, Kim Y, Han MA, Lee HY, Choi KS, Jun JK, et al. Results of colorectal cancer screening of the national cancer screening program in Korea, 2008. Cancer Res Treat. 2010;42:191–8.
15. Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112:608–12.
16. Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLoS One. 2013;8:e79292
17. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–31.
18. Fritz A, Percy C, Jack A, Shanmugarantnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology (ICD-O). 3rd edGeneva: World Health Organization; 2000.
19. Young JL Jr, Roffers SD, Ries LA, Fritz AG, Hurlbut AA. SEER summary staging manual 2000: codes and coding instructions. Bethesda, MD: National Cancer Institute; 2001.
20. Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385–91.
21. Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257–67.
22. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102.
23. Chiu SY, Chuang SL, Chen SL, Yen AM, Fann JC, Chang DC, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut. 2017;66:293–300.
24. Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218–25.
25. Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006;55:1145–50.
26. Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat. 2012;44:219–26.
27. Murphy G, Devesa SS, Cross AJ, Inskip PD, McGlynn KA, Cook MB. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer. 2011;128:1668–75.
28. Wu X, Chen VW, Martin J, Roffers S, Groves FD, Correa CN, et al. Subsite-specific colorectal cancer incidence rates and stage distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13:1215–22.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||