INTRODUCTION
Together with the discovery of alpha-fetoprotein in 1963 (1), the confirmation of carcinoembryonic antigen (CEA) in the serum of colorectal cancer patients by radioimmunoassay by Thomson et al. in 1969 (2) brought interests and investigation on the new field tumor-associated antigen that may facilitate the early detection of cancer. Different from the initial expectation, however, due to the low sensitivity and specificity for the early detection of cancer, tumor markers may be considered to be more meaningful to evaluate treatment outcomes after radical surgery or during chemotherapy (3), or the early detection of recurrence during the follow up observation (4) rather than as a diagnostically significant screening test.
CEA has been known to be a glycoprotein with minimum 5 peptide epitopes, 150,000~200,000 Daltons molecular weight (5), and particularly, serum CEA is a representative tumor maker that has been known to be elevated in almost all solid tumors such as colorectal cancer, gastric cancer, pancreatic cancer, pulmonary cancer, breast cancer, head and neck cancer (6).
The diverse positive rate of CEA in gastric cancer has been reported, from 14.8% to 37% (7~10). The normal range of serum CEA has been defined differently depending on reports, and in some studies, 2.5 ng/ml was defined as the upper limit since 90% of normal adults were included in the range (11), and for cases higher than 2.5 ng/ml, the repeat test may be recommended (12). However, serum CEA is elevated in cases with alcoholic hepatic cirrhosis (13), and it also has been known to be elevated in benign diseases, for example, chronic bronchitis, pulmonary emphysema, ulcerative colitis, gastric ulcer, atrophic gastritis (14). In addition, it has been reported to be elevated even in some healthy individuals, such as in the elderly or smokers, the difference up to approximately 3 ng/ml was shown, and it decreased to lower than 5 ng/ml within 3 to 6 months upon quitting smoking (15). And thus, considering up to 5 ng/ml as a normal range, the false positive rate in normal smokers has been reported to be 5% (14,15). Therefore, it has been known that the result value of serum CEA test should be interpreted cautiously, and it is known that a proprietary guideline of each institution performing the test for the normal range is required (12,14). Hence, the measurement of serum CEA in gastric cancer is more meaningful during the follow up examination after surgical resection or the evaluation of treatment effectiveness applying periodic or serial tests rather than as a diagnostic screening test (3,4), and the value of peripheral blood CEA measured prior to surgery as a prognostic factor is still controversial (4,8).
Radioimmunoassay is a highly sensitive and specific assay method that uses the competition between radio-labeled and unlabeled substances in an antigen-antibody reaction to determine the concentration of the unlabeled substance (serum CEA). Immunohistochemical staining method could be applied to formalin fixed tissues prepared for hematoxylin-eosin staining, its result could be obtained rapidly because the staining procedure is relatively simple, and the burden of the exposure to radiation is absent, and thus it has been used widely (16,17).
CEA expressed in gastric cancer tissues has been known to be present in the circulating blood flow through the blood vessel or the lymphatic duct, and in the current study, the relation of the elevation of CEA value measured by radioimmunometric assay in the peripheral blood of patients prior to surgery and the expression of CEA in resected primary cancer by immunohistochemical staining was evaluated, and their significance as a prognostic factor was assessed.
MATERIALS AND METHODS
1) Patients
Among patients with gastric adenocarcinoma who underwent gastrectomy from June 1998 to February 2002 in the Department of Surgery, Kyungpook National University Hospital, the study was conducted on 810 patients whose preoperative serum CEA and the expression of CEA in primary cancer tissues after surgery were examined.
There were 280 female patients and 530 male patients. The age distribution was from 24 years to 87 years, their mean age was 58.18±11.23 years, and there was no significant difference between the male and the female (male, 58.36±10.56 years; female, 57.84±12.42 years; p=0.556). Regarding surgical procedures, total gastrectomy was performed in 196 cases (24.2%), distal subtotal gastrectomy was performed in 593 cases (73.2%), and partial gastrectomy was performed in 21 cases (2.6%). Among them, D2 lymphadenectomy was performed in 801 cases (98.9%), and in the remaining 6 cases (0.7%) and 3 cases (0.4%), D1 and D3 lymphadenectomy was performed, respectively. Curative surgery was performed on 763 cases (94.2%), and palliative resection was performed on 47 cases (5.8%).
2) Methods
Serum CEA was measured by radioimmunometric assay. Prior to surgery, the blood was collected from the antecubital vein of patient, stored in a non-heparinized tube at 2~8℃, and the test was performed within 24 hours. The serum CEA level was measured by the ELSA2-CEA kit® (CIS bio international, France) using 125-I anti-CEA antibody. The reagents used for the test were stored at room temperature (18~25℃) for 30 minutes immediately prior to their use; 300 µl 125-I anti-CEA monoclonal antibody solution and 100 µl serum samples were added to the ELSA container, mixed on a Vortex, and incubated for 3 hours at 45℃. The CEA level higher than 7 ng/ml that has been known to minimize false positive rate caused by age and smoking was regarded as positive according to the manufacturer's manual.
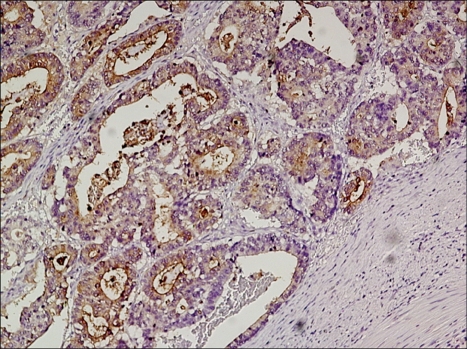
The expression of CEA in gastric cancer tissues was assessed by immunohistochemistry. Formalin-fixed paraffin-embedded tissue block was sliced to 3~4 µm thickness, and it was attached to a slide and dried. Paraffin sections were deparaffinized in xylene, and dehydrated in 100% alcohol, and then treated with 3% hydrogen peroxide in methanol to remove endogenous peroxidase activity. The sections were washed with 8:2 mixture solution (pH 7.4) of distilled water and Tris-buffered saline(TBS) Plus Tween 20® (ScyTek Laboratories, Logan, UT), immersed in citrate buffer (pH 6.0) for 2~3 minutes, and then heated in a microwave oven at 110℃ for 10 minutes to retrieve antigens, followed by cooling in room temperature for 5 minutes. Labeled Streptavidin-Biotin Peroxidase technique incorporating Monoclonal Antibody to CEA Clone SP-651® (BioGenex Laboratories, San Ramon, CA) in 1:300 dilution as primary antibody, and UltraTek HRP kit (Antipolyvalent)® (Immunotech, Canada) as secondary antibody and antibody for blocking background staining was used. Incubation with the primary antibody was done for 1 hour at room temperature. DAB solution (DakoCytomation, Denmark) was used as a chromogen, incubated for 3 minutes at room temperature, and Mayer's hematoxylin was used for counterstain. The result of immunohistochemical staining was evaluated based on the percentage of positive cells as samples without positive cells at all were Negative, less than 10% was 0+, 10-25% was 1+, 25~50% was 2+, 50~75% was 3+, more than 75% was 4+, and the result lower than 10% was classified as negative and more than 10% was classified as positive (Fig. 1). Colorectal cancer tissues were used as the positive control, and nerve tissues were used as the negative control.
Positive rates of the serum CEA and the tissue CEA expression were compared and their correlation was evaluated. Differences in positive rates according to sex and age were compared. Serum and tissue CEA positivity according to invasion depth, extent of lymph node metastasis, distant metastasis, stage, size and location of the primary tumor, gross type, histological differentiation, Lauren classification, Ming classification, and curability of surgery were evaluated. Five year survival rates and recurrence rates were compared. Recurrence rates were calculated exclusively in 763 cases who had underwent curative resection.
As TNM classification, the 1997 UICC method (18) was used. The curability of surgery was classified according to Japanese Classification of Gastric Cancer (19); Resection A or B were classified as curative resection, and resection C as noncurative resection. As the follow up observation of patients, their survival and the cause of death were assessed by the visit to outpatient clinics or telephone interview, and as the follow up examination, physical examination and various imaging tests were performed, and histological test was performed in some cases.
The median follow up period was 4.03 years. Pearson's chi-square test or Fisher's exact test was used to analyze the relationship between the serum or tissue CEA positivity and clinicopathological variables. Additionally, Kendall's tau-b method was used for the ordinal variables. Survival rates were calculated by the Kaplan-Meier method and compared by the log-rank method. As multivariate analysis, Cox's Proportional Hazards Model Analysis was used. p<0.05 was considered to be statistically significant.
RESULTS
The CEA positive rate of the serum and the primary tumor tissue was 9.3% and 91.1%, respectively, and they did not show a correlation. Regarding the serum CEA positive rate according to the expression level of CEA in primary tumor tissues, when tissue CEA was Negative, 0+, 1+, 2+, 3+, or 4+, it was 8.2%, 8.7%, 9.5%, 6.3%, 8.1%, or 11.8%, respectively, and a significant difference was not shown (p=0.532).
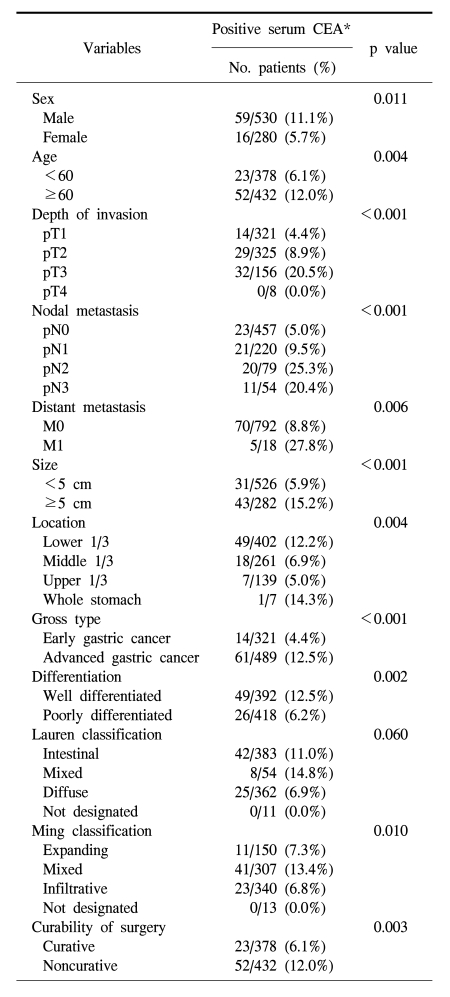
The serum CEA positive rate in the male was significantly higher than the female (11.1% vs. 5.7%; p=0.011), and the positive rate was higher in the elderly group (Table 1; p=0.004). The positive rate of serum CEA correlated with the invasion depth (p<0.001), extent of lymph node metastasis (p<0.001), presence of distant metastasis (p=0.006), stage of disease (p<0.001), tumor size (p<0.001), location (p=0.004), differentiation (p=0.002), Ming classification (p=0.010), and curability of surgery (0.003). The serum CEA positive rate was higher in intestinal type than in diffuse type, however, the difference was not significant.
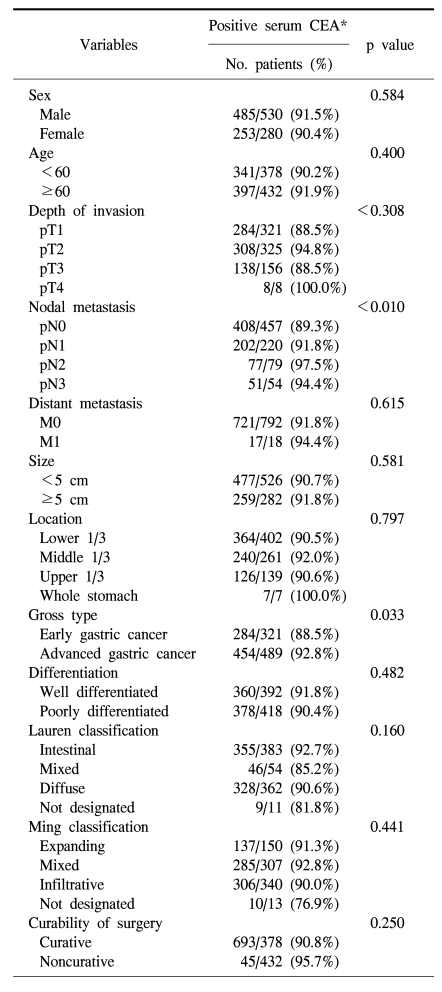
The positive rate of CEA expression in the gastric cancer tissue was higher in the male, but not statistically significant (91.5% vs. 90.4%; p=0.584), and it did not show a difference according to age (Table 2). The CEA positive rate of gastric cancer tissue was higher in macroscopically advanced cancer than early cancer (p=0.033), nonetheless, it was not a pattern increased according to the microscopic depth of invasion (p=0.308). However, it was higher in cases with lymph node metastasis (p=0.010), and as the disease stage became higher (p=0.029). The expression of CEA in the primary tumor was not associated with tumor size, Lauren's classification, and Ming's classification (Table 2).
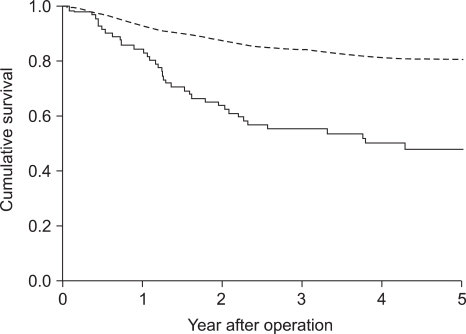
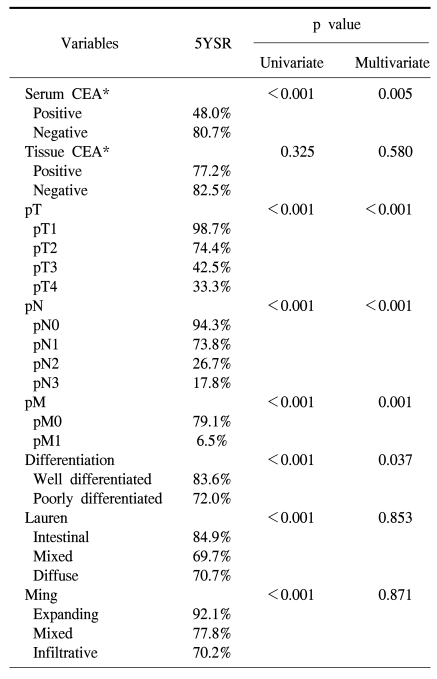
Recurrence rate after curative surgery was significantly higher in serum CEA positive cases (33.8% vs. 13.3%; p<0.001). Five-year survival rate of serum CEA positive cases (48.0%) was significantly lower than that of negative cases (80.7%; Fig 2; p<0.001), and it was also significant in multivariate analysis (Table 3). The expression of CEA in primary cancer tissues was not significantly associated with recurrence (15.3% vs. 12.9%; p=0.587), and the difference in 5-year survival rate after surgery was not significant (Table 3).
DISCUSSION
The positive rate of serum CEA has been reported to be from 14.8% to 37% depending on the standard value applied by each institution, the type of antibody used, and its sensitivity and specificity (7~10). In the current study, relatively higher cut-off level (7 ng/ml) seems to cause lower positive rate of the serum CEA (9.3%). According to age and gender, serum CEA positive rate was higher with age, and higher in the male, and thus a pattern similar to the distribution in a general population was shown (11,15).
Except pT4, the serum CEA positive rate increased according to the invasion depth, and the difference was statistically significant (p<0.001). Serum CEA was positive in none of 8 pT4 cases, which is considered to be because a small number of pT4 cases was the indication of surgery. Consequently, in comparison with pT1, pT2, or pT3, the number of patients was relatively small. It showed a significant difference according to lymph node metastasis, distant metastasis, and the final disease stage. The value of the measurement of serum CEA was proportional to the primary tumor size (20), and it has been reported in animal studies that the elevation of serum CEA was caused by the increase of the weight of primary cancer as well as the increase of the CEA production in cancer tissues (21), and in the current study, it was confirmed that as the primary tumor size became larger, the serum CEA positive cases became more abundant. It has been reported that serum CEA is not associated with the location of the primary cancer (10), however, in the current study, tumors located in the lower 1/3 of the stomach had significantly higher positive rate of serum CEA than those located in the upper 1/3 or middle 1/3. The serum and tissue CEA positive rates according to the Lauren's classification or the Ming's classification are controversial (10,17,22), and in the current study, a correlation of the expression of CEA in cancer tissues and the histological type according to the Lauren's as well as Ming's classification was not shown, nevertheless, serum CEA showed a higher positive rate in the intestinal type of the Lauren's classification than in the diffuse type, and in addition, a higher rate was detected in the expanding type of the Ming's classification. In each classification, however, mixed type had much higher CEA positive rate than other types, and this seems to cause the statistically significant difference in the serum positivity according to the Ming classification.
The positive rate of the expression of CEA in tissues was 91.1%, which shows the result concurring to the opinion that all gastric adenocarcinomas express CEA (17,20,22). Although the positive rate of the expression of CEA in primary tumor tissues was significantly higher in macroscopically advanced cancers than in early cancers (p=0.033), it was not significantly different according to the microscopic invasion depth or distant metastasis. Thus it appears that it could not reflect the overall progression level or malignant potential. In addition, tissue CEA expression was not correlated with the size or the location of the primary tumor, and this can be explained by the fact that most tumor expressed CEA regardless of its size or the disease progression. Nishida (9) reported that the expression of CEA was more frequent and stronger in the more differentiated cancers, and CEA was localized in luminal border, cytoplasm and glandular lumen in more differentiated cancers, and only in cytoplasm in less differentiated cancers. In the current study, however, tissue CEA expression was not correlated with the degree of differentiation.
The elevation of serum CEA and the expression of CEA in tissues did not show a direct correlation. It appears that the presence of CEA in the serum is dependent on the increase of the production by cancer cells, however, the increase of CEA in the peripheral blood was shown not only in several benign disease but also it was determined by the influence of several factors such as the secretion process from cancer tissues to the blood, metabolic reduction in the liver, etc. (13,20,21).
Nakane et al (23) compared preoperative serum CEA and clinicopathological findings of 865 gastric adenocarcinoma patients, and they found that preoperative serum CEA higher than 10 ng/ml was an independent prognostic factor. In a multicenter study conducted at 66 institutions, the group with a low serum CEA value prior to surgery showed a significantly higher survival rate (24). Ikeda et al (25) reported that preoperative serum CEA together with CA19-9 had a potential to be an independent prognostic factor, and in patients with elevated preoperative CEA, more comprehensive postoperative follow up or the decision of appropriate adjuvant chemotherapy would be required. On the other hand, Kodera et al (7) reported that preoperative serum CEA was not an independent prognostic factor. Concerning the expression of CEA in cancer tissues, Kojima et al (16) reported in a study on 162 cases that a higher 5-year survival rate was shown in the group of patients with tumor stained as negative or weakly positive than in the group stained as strong positive, nevertheless, contradictory results have been also reported (17). In the current study, the expression of CEA in tissues was not associated with the recurrence rate as well as 5-year survival rate. On the other hand, in the group with negative preoperative serum CEA, 5-year survival rate was significantly higher (p<0.001), and a significant difference was detected also by multivariate analysis (p=0.005). And, the recurrence rate after curative surgery was significantly higher in the serum CEA positive group (p<0.001).
CONCLUSION
The preoperative serum CEA positivity had significant correlation with the disease progression and recurrence after curative resection. In addition, it showed significantly poor survival both in the univariate and in the multivariate analysis, and thus, it could be considered as an independent prognostic factor in gastric adenocarcinoma patients. Consequently, more intensive follow up evaluation or adjuvant chemotherapy is recommended in patients with increased preoperative serum CEA levels. Despite the positive correlation with the lymph node metastasis, tissue CEA expression, however, had little information on the disease progression or survival. CEA expression in gastric cancer tissue was present in most cases, and had no prognostic significance.