AbstractPurposeWe investigated 18F-fluorodeoxyglucose positron emission tomography (PET)-derived parameters as prognostic indices for disease progression and survival in locally advanced nasopharyngeal carcinoma (NPC) and the effect of high-dose radiotherapy for a subpopulation with PET-based poor prognoses.
Materials and MethodsNinety-seven stage III and Iva-b NPC patients who underwent definitive treatment and PET were reviewed. For each primary, nodal, and whole tumor, maximum standardized uptake value, metabolic tumor volume, and total lesion glycolysis (TLG) were evaluated.
ResultsBased on the C-index (0.666) and incremental area under the curve (0.669), the whole tumor TLGwas the most useful predictorfor progression-free survival (PFS); thewhole tumor TLG cut-off value showing the best predictive performance was 322.7. In multivariate analysis, whole tumor TLG was a significant prognostic factor for PFS (hazard ratio [HR], 0.3; 95% confidence interval [CI], 0.14 to 0.65; p=0.002) and OS (HR, 0.29; 95% CI, 0.11 to 0.79; p=0.02). Patients with low whole tumor TLG showed the higher 5-year PFS in the subgroup for only patients receiving intensity modulated radiotherapy (77.4% vs. 53.0%, p=0.01). In the subgroup of patients with high whole tumor TLG, patients receiving an EQD2 ≥ 70 Gy showed significantly greater complete remission rates (71.4% vs. 33.3%, p=0.03) and higher 5-year OS (74.7% vs. 19.6%, p=0.02).
IntroductionRadiotherapy (RT) with or without chemotherapy is known to improve treatment outcomes including local control and survival in nasopharyngeal carcinoma (NPC) [1,2]. Even in locally advanced stage III-IVb NPC, a survival rate of more than 70% and loco-regional control rate of more than 90% have been reported [3,4]. Nevertheless, local, regional, and distant failure (DF) after completion of definitive treatment has been reported in 5.9%-11.6%, 7.4%-10%, and 14.7%-20.9%, respectively [5,6]. This was the impetus to investigate a predictive index for disease progression through the recent development of an RT modality and improved imaging techniques.
18F-fluorodeoxyglucose positron emission tomography 18F-FDG-PET is useful in patients with head and neck malignancy for tumor staging, including locally advanced NPC [7-9]. Several studies have shown that PET-derived parameters, including the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG), could have prognostic value in prediction of disease progression and survival in patients with head and neck cancer. In NPC, many studies have also found correlation of higher SUVmax, MTV, and TLG with poorer treatment outcomes [10-12]. However, few studies have investigated prognostic value of several PET-derived parameters for only locally advanced NPC [10], and no study has evaluated the survival benefit of a higher RT dose in locally advanced NPC showing higher PET-derived parameters predicting a poor prognosis.
Therefore, this study was conducted to examine the usefulness of PET-derived parameters as prognostic indices for prediction of disease progression and survival in patients with locally advanced NPC. We also examined the question of whether high-dose RT could improve treatment outcomes in a subgroup with poor prognoses based on PET-derived parameters.
Materials and Methods1. Patient selectionThis retrospective review was approved by the Institutional Review Board. Between 2004 and 2013, 156 patients diagnosed with stage III or IVa-b NPC underwent definitive treatment at our institution. Among them, patients with a good Eastern Cooperative Oncology Group (ECOG) performance status and normal hepatic, renal, and bone marrow function were eligible. Forty-nine patients with another primary malignancy, past history of previous RT or chemotherapy, and those who did not undergo 18F-FDG-PET before definitive RT were excluded. Ten patients whose 18F-FDG-PET images were unavailable were also exclu-ded and the medical records of 97 patients were reviewed. All patients had NPC confirmed by biopsy at the primary site. All biopsy specimens were classified according to three categories based on the World Health Organization (WHO) criteria as follows: keratinizing carcinoma, type I; non-keratinizing carcinomas, type II; and undifferentiated carcinoma, type III. All patients were staged based on the system developed by the 7th American Joint Committee on Cancer [13].
2. RadiotherapyRT was performed using 3-dimensional conformal RT (3D-CRT) or intensity modulated radiotherapy (IMRT). 3D-CRT was delivered as 1.8-Gy daily fractions using 6-MV or 10-MV photon beams 5 days a week for a total gross tumor volume (GTV) dose of 70.2 Gy by a linear accelerator. The clinical target volume (CTV) was administered at a dose of 59.4 Gy. A radiation dose of 45.0 to 54 Gy was delivered for elective nodal irradiation. Two lateral and parallel opposing fields involved the nasopharynx, skull base, and upper part of the neck. The lower neck was treated with an anterior single field with midline shielding. After 45 Gy, spinal cord shielding was performed. The IMRT technique, including simulation, planning, and dose constraints of organs at risk, was described previously [14]. The GTV received a total dose of 69.96 Gy in daily fractions of 2.12 Gy; the CTV received 59.4 Gy in 1.8-Gy daily fractions and the target volume for elective nodal irradiation received 56.1 Gy in 1.7-Gy daily fractions.
3. ChemotherapyConcurrent chemoradiation (CCRT) or induction chemotherapy (IC) followed by CCRT was performed for most patients [14]. Concurrent chemotherapy was administered with RT as weekly cisplatin 30 mg/m2 (DDP), weekly cisplatin 20 mg/m2 plus 5-fluorouracil (5-FU) 750 mg/m2 (FP), and 5-FU 750 mg/m2 plus Taxotere 70 mg/m2 plus cisplatin 75 mg/m2 every third week (FTP). At our institution before 2006, chemotherapy regimen decisions were based on the physicians’ discretion, with a preference for FTP or FP regimens for advanced stages including T3-4 and N1-3. Since 2006, concurrent cisplatin has been the standard treatment for NPC. The IC regimen consisted of cisplatin 75 mg/m2 and 5-FU 1,000 mg/m2 for 5 days (on days 1-5) repeated every 3 weeks, and followed by CCRT regimens beginning 3 weeks after the third course of IC.
4. 18F-FDG-PETFor all patients, 18F-FDG/PET scans were performed using a dedicated PET/computed tomography (CT) scanner (Discovery STE, GE Healthcare, or Biograph TruePoint 40, Siemens Healthcare, Malvern, PA), within 1 to 2 weeks before definitive treatment. The detailed protocols for measuring blood glucose concentrations, determining the quantity of injected 18F-FDG, the low-dose CT scan, the PET scan, PET data reconstruction, and the contrast-enhanced CT scan after completion of PET acquisition have been described previously [15]. For each primary, lymph nodal metastatic, and whole tumor, the SUVmax, mean SUV, MTV, and TLG were measured using the PETedge tool in MIMvista software (MIMvista Corp., Cleveland, OH) according to the protocol described by Liao et al. [16]. The PET parameters for lymph nodal metastatic tumors were calculated only for patients showing lymph nodal metastasis. SUVmax was calculated as [(decay-corrected activity/tissue volume)/(injected dose/body weight)] and MTV was defined as total tumor volume with an SUV of 2.5 or greater. TLG was defined as the product of mean uptake and metabolic volume. TLG was calculated as [(mean SUV)×(MTV)] [17]. After contouring the tumor using the PETedge tool, volumes of interest (VOIs) were automatically generated from spatial derivatives to locate the tumor surface. The estimated VOIs were manually adjusted using a 2-D “ball” contouring tool.
5. Follow-up, response evaluation, and patterns of failureAfter completion of treatment, patient follow-up assessments and follow-up imaging studies were performed at 1, 3, and 6 months after RT, and then every 6 months until 2 years after RT, and annually thereafter. Treatment responses were evaluated by recording a history, performing a physical examination, nasopharyngoscopy, and imaging studies, such as magnetic resonance imaging and CT, at 3 months after completion of all treatments. Complete remission (CR) was defined as a 100% decrease in gross tumor from a clinical evaluation or radiologic images. Partial response (PR) and progressive disease (PD) were defined as a ≥ 50% decrease and > 25% increase of the primary gross tumor, respectively; other cases were categorized as stable disease (SD). Locoregional failure (LRF) was defined as recurrence or progression at the primary site and neck nodal regions and DF as metastasis outside the primary site and neck nodal regions. LRF or DF was investigated from the date of diagnosis until the date of the first failure.
6. Statistical analysisThe primary endpoint was progression-free survival (PFS). PFS was calculated from the treatment start date to the date of progression, relapse, death from any cause, or last contact. Overall actuarial survival (OS) was calculated from the treatment start date to the date of death or the last follow-up. Loco-regional or distant failure-free survival (LRFFS or DFFS) was recorded as the treatment start date to the date of LRF occurring at any time before outfield failure or death from any cause for LRFFS or to the date of the first DF with or without LRF at any time before or after LRF or death from any cause for DFFS. PFS, OS, LRFFS, and DFFS were calculated using the Kaplan-Meier method, and compared using the log-rank test. Cox proportional hazards model was performed using stepwise backward selection for all factors in univariate and multivariate analyses for prognostic factors. The hazard ratio (HR) is given with 95% confidence intervals (95% CIs). The C-index and incremental area under the curve (iAUC) were calculated using Cox’s proportional hazards model to determine the most useful PET parameter for prediction of disease progression. The C-index is defined as the probability of concordance between prediction and outcomes among all possible pairs. The concordance (C) statistics or C-index is calculated as the sum of concordance values divided by all possible pairs [18]. The Contal and O’Quigley method, based on the log-rank test, was used to determine the cut-point for the most useful PET parameter [19]. The optimal cut-point is determined using an algorithm maximizing the HR. Patients were divided into two groups according to cut-point. Differences in nominal variables were compared using Pearson’s chi-square test or Fisher exact test; continuous variables were analyzed using the Mann-Whitney U test and t test. Propensity-matching analysis was performed to adjust for clinical factors that were different between two groups based on cut-off values. p < 0.05 were considered significant. SPSS ver. 20.0.0 (IBM Co., Armonk, NY), SAS ver. 9.2 (SAS Institute Inc., Cary, NC), and R statistical software ver. 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Results1. Patient and treatment characteristicsPatient and treatment characteristics are listed in Table 1. Median age was 50 years (range, 13 to 75 years). Forty-one patients (42.3%) had an ECOG performance status of 0. Sixty-nine patients (71.1%) were male. Non-keratinizing and undifferentiated carcinoma were noted in 49 (50.5%) and 42 patients (43.3%), respectively. There were 53 advanced T (T3-4) patients (54.6%) and 90 N+ stage patients (92.8%). Seventy-four patients (76.3%) underwent IMRT. The median dose of equivalent dose in 2 Gy fractions (EQD2) (α/β ratio=10) was 70.7 (32.1-73.8). Chemotherapy was administered in 92 patients (94.9%).
2. Treatment outcomesThe median follow-up duration among surviving patients was 47 months (range, 8 to 127 months). The 5-year PFS and OS were 64.9% and 75.2%, respectively. LRF and DF were observed in 11 (21.2%) and 19 patients (19.6%), respectively. CR was reported in 75 patients (77.3%), PR in 16 (16.5%), SD in 4 (4.1%), and PD in 2 (2.1%) at 3 months following treatment completion.
3. 18F-FDG-PET parametersThe average SUVmax for whole tumors and the primary tumor was 13.42±6.47 and 12.43±6.39, respectively. The average MTV for whole tumors and the primary tumor was 50.9±37.72 mL and 25.31±23.26 mL, respectively. The average TLG for whole tumors and the primary tumor was 310.81± 276.37 and 173.91±197.33, respectively. For stage N1-3 patients, the mean SUVmax, MTV, and TLG of metastatic lymph nodes were 10.66±6.2, 27.58±36.96 mL, and 147.55±227.59, respectively.
4. Usefulness of 18F-FDG-PET for predicting disease progressionThe C-index was calculated to determine the most useful parameter for predicting disease progression among nine 18F-FDG-PET parameters. The TLGs for the whole tumor and primary tumor were the most valuable PET parameters (C-index, 0.666) (Supplementary Table 1). Thereafter, using Contal and O’Quigley’s method, the cut-points of whole tumor TLG and primary tumor TLG were determined as 322.7 and 123.1 (Supplementary Table 2). In comparison of iAUC values, whole tumor TLG (iAUC, 0.669; 95% CI, 0.786 to 0.78) was better at predicting disease progression than primary tumor TLG. Next, the patients were divided into the low whole tumor TLG group (< 322.7) and the high whole tumor TLG group (≥ 322.7).
Differences in patient characteristics were assessed between the low and high whole tumor TLG groups (Table 2). The high whole tumor TLG group included more advanced stage Iva-b patients than the low whole tumor TLG group with borderline significance (p=0.05). The whole tumor volume of high whole tumor TLG was significantly higher than that of low whole tumor TLG (p < 0.001). There was no difference in other clinical factors between the groups. Examination of differences in treatment characteristics, treatment response, and patterns of failure according to whole tumor TLG (Table 3) showed no significant difference in treatment characteristics between the groups. The low whole tumor TLG group included significantly more patients showing CR (87.5% vs. 57.6%, p=0.001) and lower LRF rate (6.3% vs. 21.2%, p=0.04) than the high whole tumor TLG group. However, despite showing a significant trend, there was no difference in DF (Table 3).
Kaplan-Meier curves demonstrated that there was a survival difference according to whole tumor TLG (Fig. 1). The low whole tumor TLG group showed significantly higher 5-year PFS (77.0% vs. 43.0%, p < 0.001), OS (85.7% vs. 54.0%, p=0.003), LRFFS (77.0% vs. 49.1%, p=0.001), and DFFS (81.6% vs. 60.3%, p=0.012) than the high whole tumor TLG group (Fig. 1A-D). Due to the era of IMRT for NPC, survival difference was analyzed according to whole tumor TLG in the subgroup for patients receiving only IMRT (Fig. 2). Similar to the result for whole patients, the low whole tumor TLG group also showed a significantly higher 5-year PFS rate (77.4% vs. 53.0%) than the high whole tumor TLG group (p=0.01) (Fig. 2A), while 5-year OS rate of the low whole tumor TLG group was not significantly higher than that of the high whole tumor TLG group (p=0.254) (Fig. 2B). The 5-year LRFFS rate of the low whole tumor TLG group (77.4%) was significantly higher than that of the high whole tumor TLG group (53.0%, p=0.01) (Fig. 2C). The low whole tumor TLG group had a higher 5-year DFFS rate than the high whole tumor TLG group with a statistically significant trend (84.6% vs. 65.0%, p=0.06) (Fig. 2D).
5. Propensity-matching analysisBecause there was a difference in TNM staging between high and low whole tumor TLG groups, propensity-matching analysis was performed to adjust for TNM staging between two groups. After propensity-matching analysis, all clinical factors, including TNM staging, were balanced between two groups, except whole tumor volume (40.6 vs. 78.3, p=0.002) (Table 4). There were no differences in treatment characteristics between the two groups (Table 5). The high whole tumor TLG group showed a significantly lower CR rate than the low whole tumor TLG group (57.6% vs. 84.8%, p=0.01) (Table 5). The high whole tumor TLG group also had more LRF and DF, although without statistical significance (Table 5). On Kaplan-Meier curves, the low TLG group showed significantly better PFS (73.5% vs. 43.0%, p=0.009), LRFFS (73.5% vs. 42.1%, p=0.015), and DFFS (77.6% vs. 60.3%, p=0.048) than the high TLG group (Fig. 3A-C). Although OS for the low TLG group was higher than that for the high TLG group, the difference was not significant (69.7% vs. 54.0%, p=0.161) (Fig. 3D).
6. Prognostic factor analysesIn univariate analysis, whole tumor TLG (p=0.001), ECOG performance status (p=0.007), TNM staging (p=0.002), and tumor volume (p=0.005) showed significant association with PFS (Table 6). In multivariate PFS analysis, whole tumor TLG (low vs. high, p=0.002), TNM staging (III vs. IVa-b, p=0.01), and chemotherapy (concurrent vs. induction vs. none, p=0.01) were independent significant prognostic factors. As shown in Table 6, in the univariate analysis for OS, ECOG performance status (p=0.03), T stage (p=0.02), TNM staging (p=0.02), RT modality (p=0.04), and whole tumor TLG (p=0.006) were significant factors. In the multivariate analysis, whole tumor TLG (low vs. high, p=0.02), T stage (T1-2 vs. T3-4, p=0.03), and RT modality (IMRT vs. 3D-CRT, p=0.03) were independent prognostic factors that significantly influenced OS.
7. Subgroup analysis according to TLGTo examine the effect of a higher EQD2 in patients with high whole tumor TLG values, a subgroup analysis was performed for the high whole tumor TLG patients. In a total of 33 high whole tumor TLG patients, 21 patients received an EQD2 of at least 70 Gy. A higher CR rate was observed in patients receiving an EQD2 ≥ 70 Gy (p=0.03) (Table 7). There was no difference in patterns of failure according to EQD2. In the Kaplan-Meier analyses of PFS, patients who received an EQD2 ≥ 70 Gy showed a higher 5-year PFS (58.9% vs. 16.7%) than those who received an EQD2 < 70 Gy, although it was not significant (Fig. 4A). However, significantly higher 5-year OS (74.7% vs. 19.6%, p=0.02) (Fig. 4B) was observed for patients who received an EQD2 ≥ 70 Gy.
DiscussionBecause TLG and MTV are volumetric PET-derived parameters, they are generally considered more optimal for reflecting tumor metabolic burden and for prediction of treatment outcome than SUVmax [17,20]. While MTV is the tumor volume showing PET uptake over a set threshold, TLG is representative of the metabolic activity of the whole tumor lesion; therefore, we believe that TLG is a better predictor of disease progression than MTV, and was significantly most powerful prognostic factor for PFS, OS, LRFFS, and DFFS of locally advanced NPC in this study.
In our findings, the high whole tumor TLG group had significantly higher whole tumor volume than the low whole tumor TLG group. TLG is a PET parameter that considers tumor burden, as well as metabolic activity [17]. We also observed that SUVmax and MTV for whole tumors were also significantly higher in the high whole tumor TLG group (SUVmax 10.7 vs. 17.3, MTV 32.2 vs. 68.7, both p < 0.001) (Table 2). Thus, we discerned that the high-TLG group would naturally show higher tumor volumes, which would not be a confounding factor in this study.
Most studies on the prognostic significance of PET-derived parameters for NPC included all stages of NPC, from early to advanced stages [11,12]. However, because advanced stages are significantly associated with higher PET parameters [12,21], and there are differences in treatment strategy and patterns of failure according to clinical staging, we consider that a study including only advanced stage cases would be appropriate for investigating the clinical usefulness of PET parameters as prognostic predictors. In 2009, Xie et al. [10] demonstrated that patients having tumors with a lower SUVmax had significantly better 5-year OS (p=0.0187) and disease-free survival (p=0.0163) than patients with a higher SUVmax in locally advanced NPC. In a recent study, Chan et al. [22] showed that a whole tumor TLG value ≥ 330 independently predicted OS (p=0.0014) and disease-free survival (p=0.0005) in locally advanced NPC patients. Similarly, in our findings, a whole tumor TLG of ≥ 322.7 showed independent association with OS and PFS. Therefore, we suggest that whole tumor TLG could be an optimal predictor of disease progression and prognosis for locally advanced NPC patients only.
Despite significant improvement in the oncologic outcomes of locally advanced NPC with the development of radiation technologies, loco-regional and DF are still major obstacles preventing improved outcomes. Given the findings from several previous studies, we consider that an evolution of therapeutic strategies is necessary and would improve oncologic outcomes in NPC patients with PET-based poor prognoses who show higher MTV and TLG values. Therefore, in this study we performed a subgroup analysis of NPC patients with high whole tumor TLG values. In the subgroup analysis, patients with high whole tumor TLG values who received higher EQD2 RT showed an improved OS with statistical significance, while EQD2 was not related to OS among all enrolled patients. Therefore, we suggest that high-dose RT could help improve survival in a subpopulation with PET-based poor prognoses. However, conduct of an additional prospective study would be necessary.
Although altered fractionation has been investigated in conjunction with concurrent chemotherapy in recent decades, the risk of serious radiation-induced neurovascular toxicity has resulted in a narrow therapeutic ratio. Using IMRT, several studies on sequential or simultaneous integrated boost have reported excellent outcomes, with low rates of radiation-induced toxicity. However, approximately 10% of temporal lobe necrosis using a daily dose of 2.16-2.34 Gy/fraction was observed [23,24]. The Radiation Therapy Oncology Group (RTOG) 0225, using a schedule of 70 Gy/33 fractions in 2.12 Gy/fraction, has demonstrated excellent survival without severe brain damage [6]: grade 1 brain late toxicity was observed in only two patients. Current National Comprehensive Cancer Network (NCCN) guidelines recommend a total of 66 or 70 Gy in 30 fractions in definitive RT and 70-70.2 Gy per a fractional dose of 1.8-2 Gy in CCRT [25]. Considering RTOG 0225 and NCCN guidelines, > 70 Gy of EQD2 (α/β ratio=10) was considered a high-dose RT in our study.
Special consideration is required when interpreting our findings because of some limitations. First, this study was retrospective in design. Second, because PET is not a fundamental or mandatory diagnostic tool in the staging work-up of NPC, PET was not performed for all NPC patients at our institution, possibly inducing selection bias. Third, because of the long duration of the treatment period from 2004 to 2013, the treatment characteristics showed heterogeneity. In particular, in the era of IMRT for NPC, patients enrolled in this study received 3D-CRT as well as IMRT. However, we performed a reasonable statistical analysis considering our above-stated limitations, and suggest that our findings could provide sufficient evidence for the implementation of a future prospective study.
ConclusionIn conclusion, our findings demonstrated that whole tumor TLG was most useful among several PET-derived parameters for prediction of disease progression, and that whole tumor TLG was a significant prognostic factor for PFS, OS, LRFFS, and DFFS. Of particular importance, this study suggests that higher EQD2 RT could improve treatment outcomes in NPC patients with high whole tumor TLG values, despite the subgroup analysis including a small number of patients. A well-designed prospective study to compare the effect of TLG-based high-dose RT to conventional RT in locally advanced NPC is needed to examine the survival benefit of high-dose RT in high TLG patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Fig. 1.Kaplan-Meier curves demonstrate the survival difference between high and low total lesion glycolysis (TLG) of the whole tumor. (A) Progression-free survival. (B) Overall survival. (C) Loco-regional failure-free survival. (D) Distant failure-free survival. 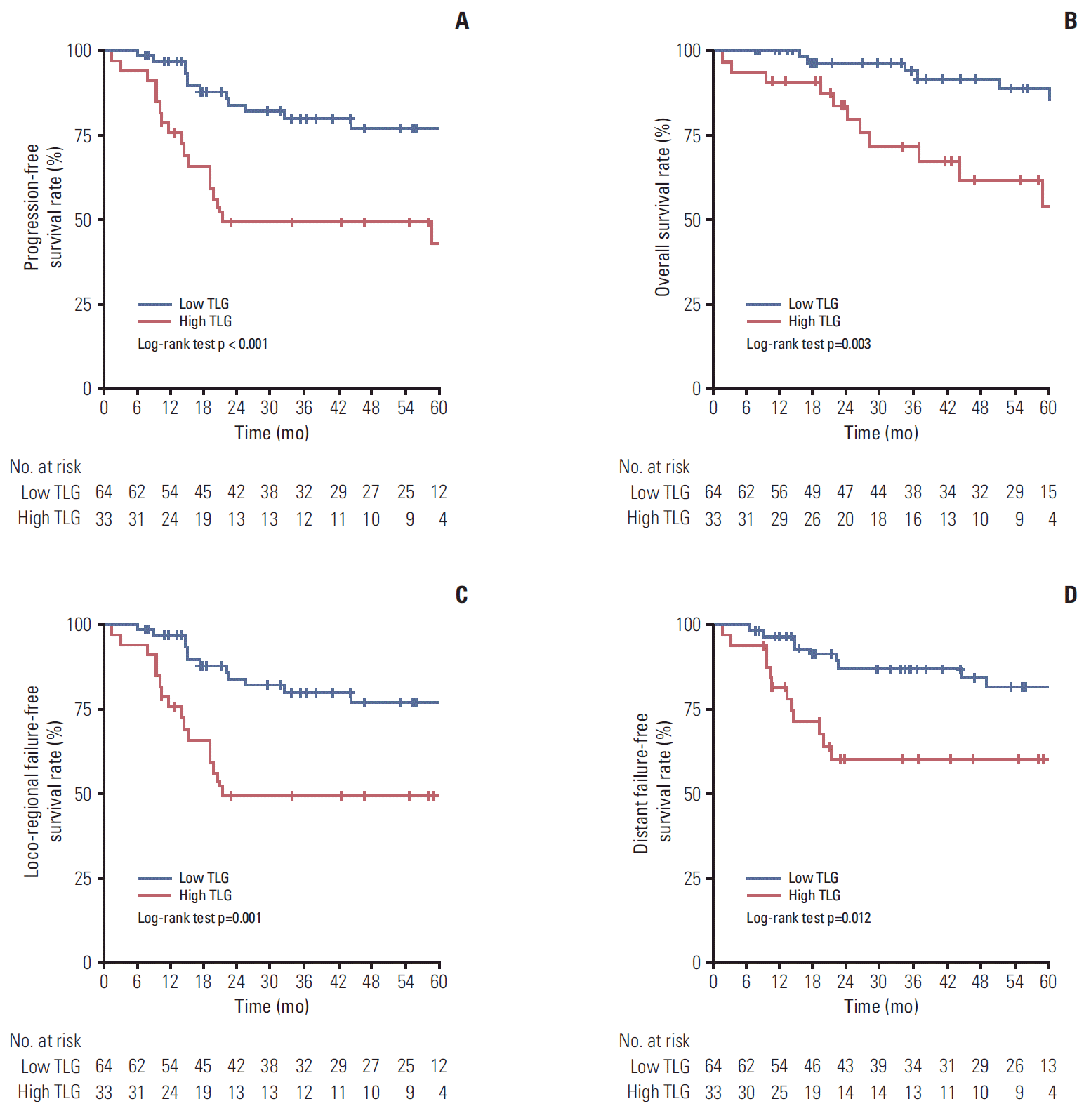
Fig. 2.Kaplan-Meier curves of progression-free survival (A), overall survival (B), loco-regional failure-free survival (C), and distant failure-free survival (D) for subgroup of the only patients receiving intensity modulated radiotherapy. TLG, total lesion glycolysis. 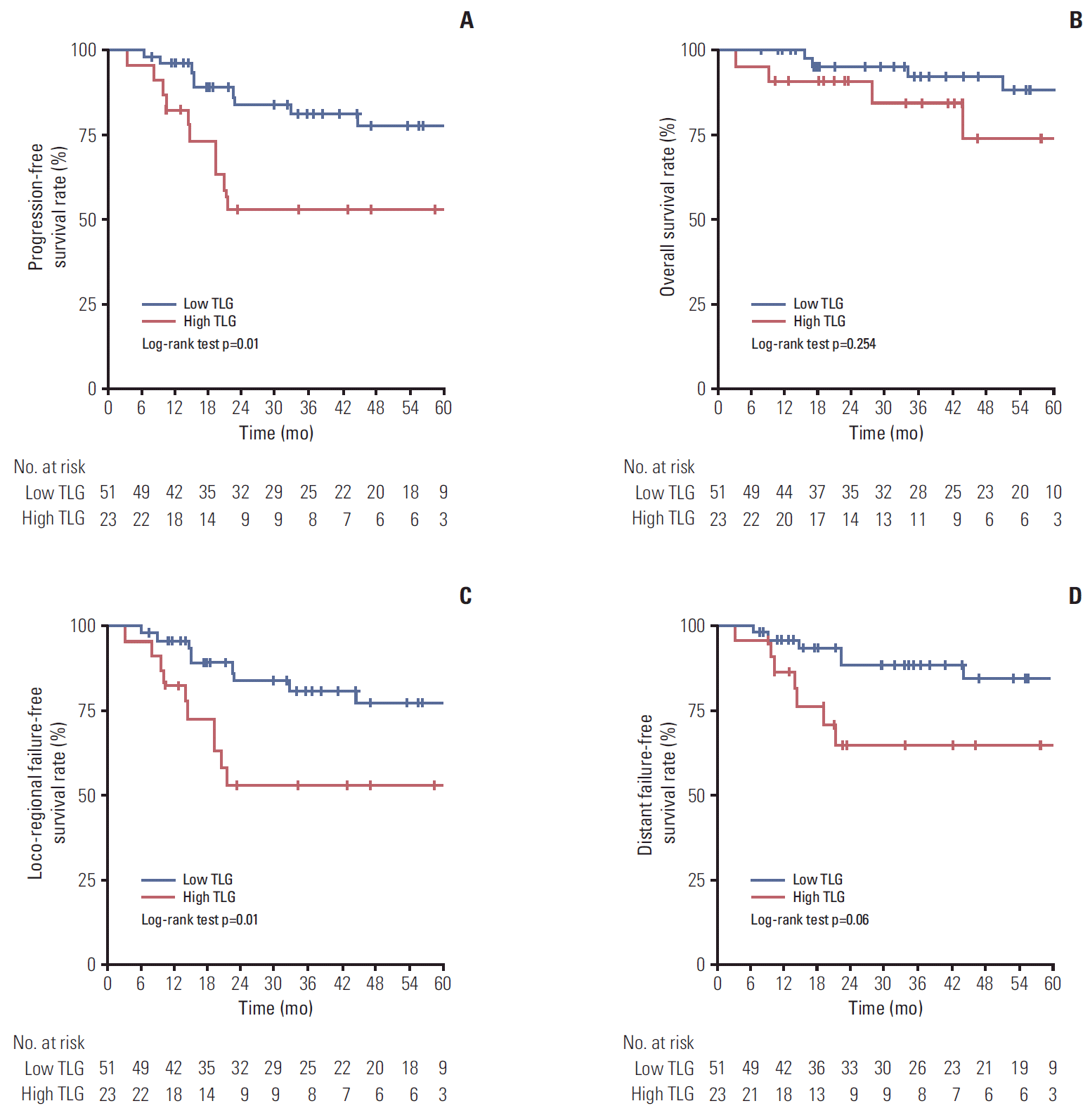
Fig. 3.Kaplan-Meier curves demonstrate survival differences after propensity-matching analysis to adjust for differences in TNM staging between high and low total lesion glycolysis (TLG) of the whole tumor. (A) Progression-free survival. (B) Locoregional failure-free survival. (C) Distant failure-free survival. (D) Overall survival. 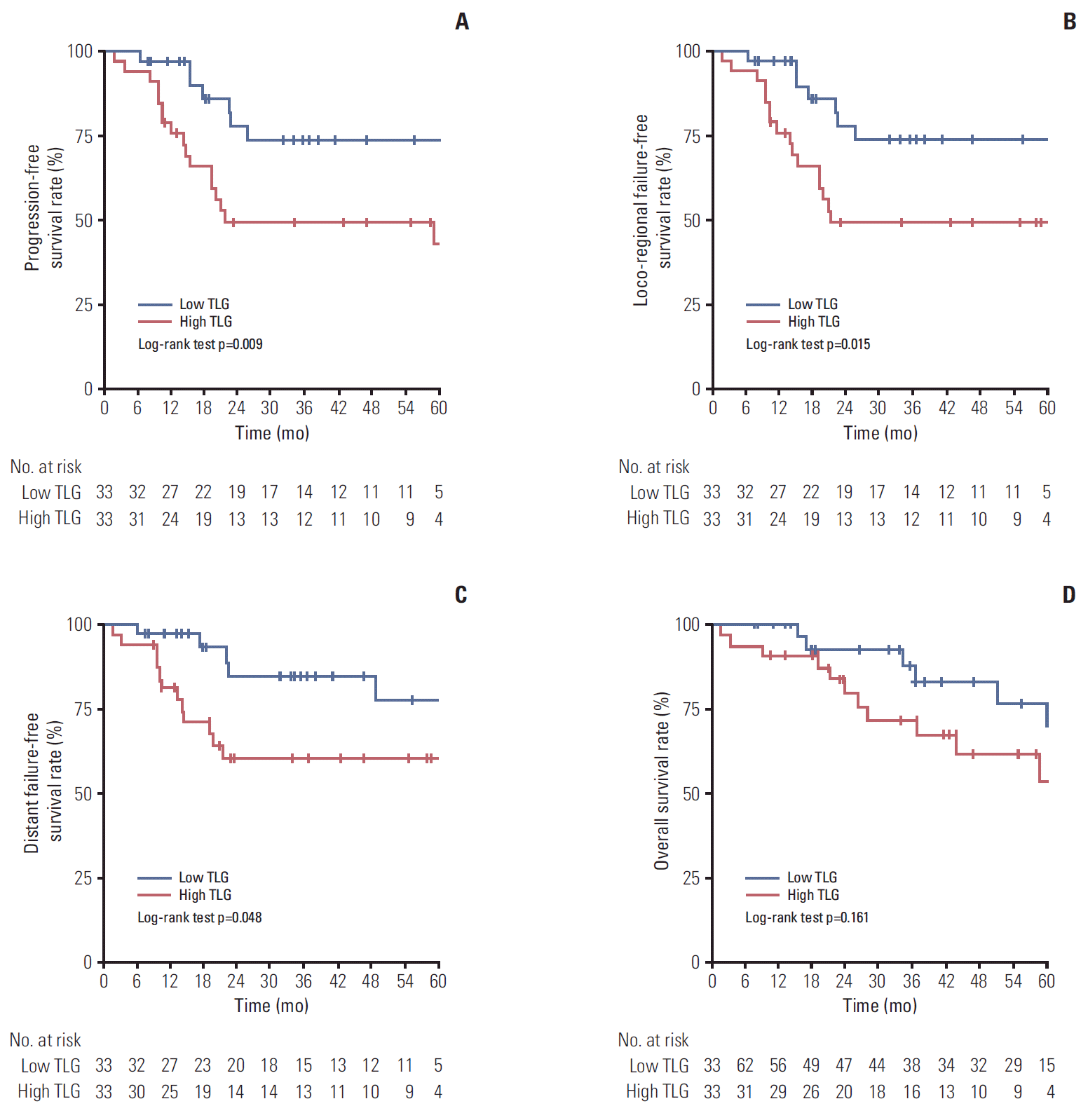
Fig. 4.Kaplan-Meier curves demonstrate the differences in progression-free survival and overall survival according to equivalent dose in 2 Gy fractions (EQD2) in subgroup of patients with high total lesion glycolysis of the whole tumor. (A) Progression-free survival. (B) Overall survival. 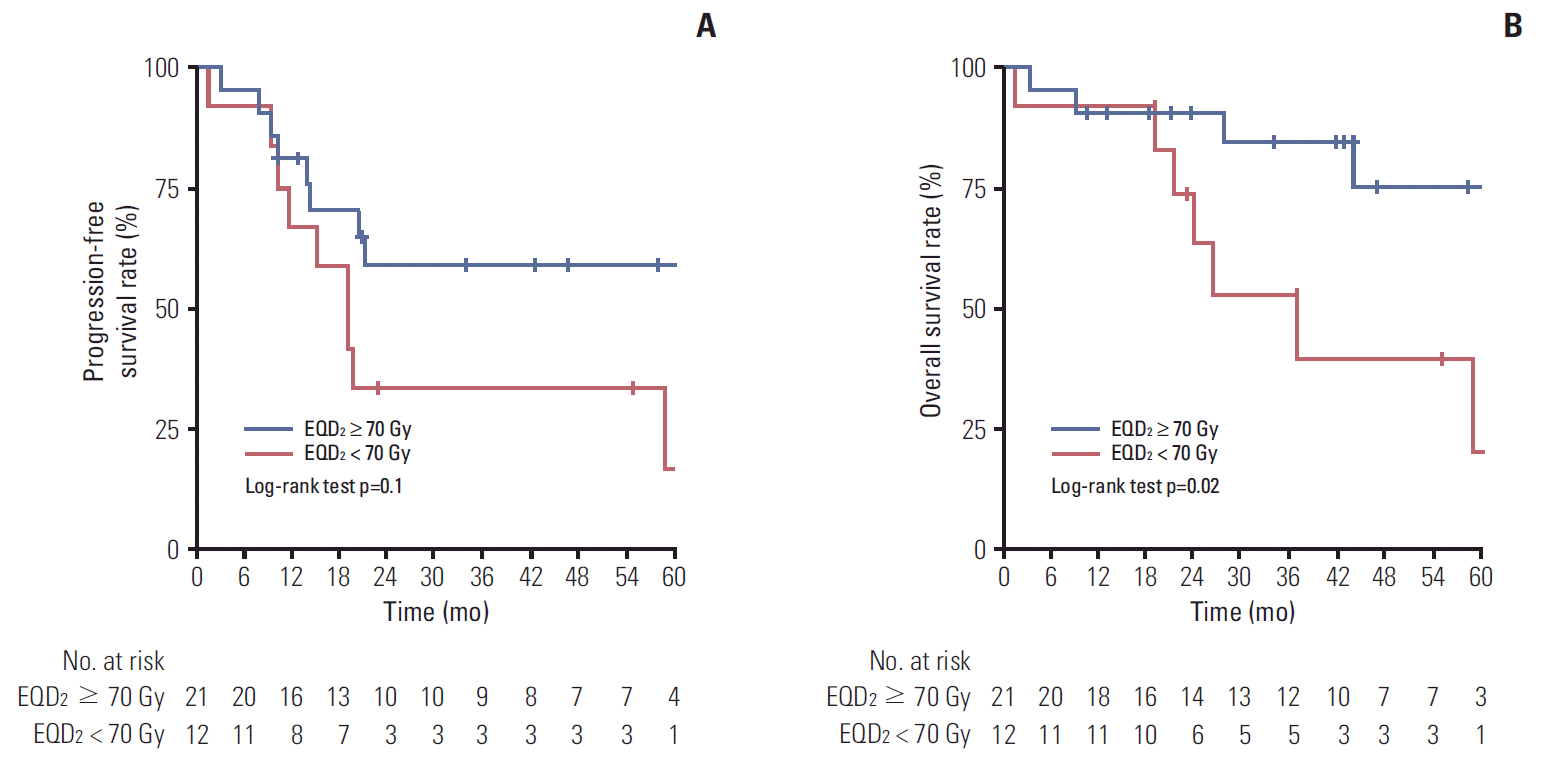
Table 1.Patient and treatment characteristics Table 2.Clinical factors according to 18F-FDG-PET parameters Table 3.Treatment characteristics, treatment response, and patterns of failure according to TLG Table 4.Clinical factors based on TLG after propensity-matching analysis
Table 5.Treatment, response, and patterns of failure based on TLG after propensity-matching analysis Table 6.Prognostic factor analyses using Cox’s regression method with backward selection for progression-free survival and overall survival HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; WHO, World Health Organization; TNM, tumor-node-metastasis; IMRT, intensity-modulated radiation therapy; 3D-CRT, 3-Dimensional conformal radiation therapy; EQD2, equivalent dose in 2 Gy fractions; TLG, total lesion glycolysis for whole tumor. Table 7.Relation between treatment response and EQD2 (α/β ratio=10) in patients having high whole tumor TLG References1. Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161–8.
2. Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56.
3. Ng WT, Lee MC, Hung WM, Choi CW, Lee KC, Chan OS, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:420–8.
4. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–7.
5. Wu F, Wang R, Lu H, Wei B, Feng G, Li G, et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiother Oncol. 2014;112:106–11.
6. Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–90.
7. Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, et al. Positron emission tomography with [18F]fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J Clin Oncol. 2010;28:1190–5.
8. Chang JT, Chan SC, Yen TC, Liao CT, Lin CY, Lin KJ, et al. Nasopharyngeal carcinoma staging by (18)F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2005;62:501–7.
9. Liu FY, Lin CY, Chang JT, Ng SH, Chin SC, Wang HM, et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. J Nucl Med. 2007;48:1614–9.
10. Xie P, Yue JB, Fu Z, Feng R, Yu JM. Prognostic value of 18F-FDG PET/CT before and after radiotherapy for locally advanced nasopharyngeal carcinoma. Ann Oncol. 2010;21:1078–82.
11. Hung TM, Wang HM, Kang CJ, Huang SF, Liao CT, Chan SC, et al. Pretreatment (18)F-FDG PET standardized uptake value of primary tumor and neck lymph nodes as a predictor of distant metastasis for patients with nasopharyngeal carcinoma. Oral Oncol. 2013;49:169–74.
12. Chang KP, Tsang NM, Liao CT, Hsu CL, Chung MJ, Lo CW, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–8.
13. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3rd. AJCC cancer staging manual. 7th edNew York: Springer; 2010.
14. Kim JW, Cho JH, Keum KC, Kim JH, Kim GE, Lee JY, et al. IMRT with simultaneous integrated boost and concurrent chemotherapy for nasopharyngeal cancer: plan evaluation and treatment outcome. Jpn J Clin Oncol. 2012;42:1152–60.
15. Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using 18F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–8.
16. Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38.
17. Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904.
18. Tripepi G, Jager KJ, Dekker FW, Zoccali C. Statistical methods for the assessment of prognostic biomarkers (Part I): discrimination. Nephrol Dial Transplant. 2010;25:1399–401.
19. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–70.
20. Ryu IS, Kim JS, Roh JL, Lee JH, Cho KJ, Choi SH, et al. Prognostic value of preoperative metabolic tumor volume and total lesion glycolysis measured by 18F-FDG PET/CT in salivary gland carcinomas. J Nucl Med. 2013;54:1032–8.
21. Chan WK, Mak HK, Huang B, Yeung DW, Kwong DL, Khong PL. Nasopharyngeal carcinoma: relationship between 18F-FDG PET-CT maximum standardized uptake value, metabolic tumour volume and total lesion glycolysis and TNM classification. Nucl Med Commun. 2010;31:206–10.
22. Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011;32:989–96.
23. Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117:1874–83.
24. Bakst RL, Lee N, Pfister DG, Zelefsky MJ, Hunt MA, Kraus DH, et al. Hypofractionated dose-painting intensity modulated radiation therapy with chemotherapy for nasopharyngeal carcinoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2011;80:148–53.
25. National Comprehensive Cancer Network. NCCN clinical practice guidelines in Oncology: head and neck cancers [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; 2015. [cited 2015 Oct 1]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||