INTRODUCTION
Metastasis of ovarian carcinoma is usually confined to the peritoneal cavity at presentation and throughout its course in approximately 85% of the patients. Gastrointestinal involvement is most often superficial, and transmural invasion is less common. Gastric involvement is extremely rare at presentation and during the course of ovarian carcinoma. To the best of our knowledge, only one case of intramural gastric metastasis of recurrent epithelial ovarian carcinoma has been reported in the English literature (1). We present here a case of primary ovarian carcinoma with gastric metastasis that mimicked gastrointestinal stromal tumor (GIST), and this was diagnosed after the patient underwent gastric antrectomy.
CASE REPORT
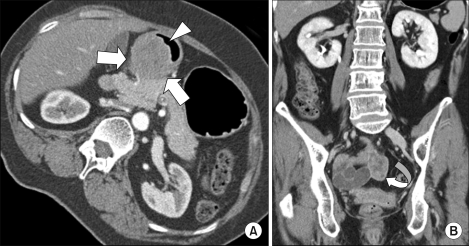
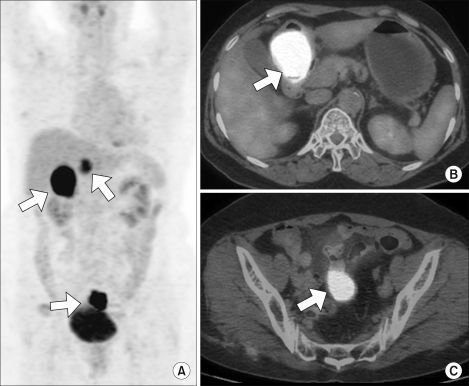
A 55-years-old gravida 4 para 2 woman presented with epigastric pain and fullness for one month. The patient had no epigastric soreness, weight loss, hematemesis or melena. She then underwent gastroduodenoscopy. On gastroduodenoscopy, a submucosal mass and a clear round deep ulceration on the mass was noted on the gastric antrum. The submucosal mass caused partial obstruction of the antrum (Fig. 1). The biopsies showed normal gastric mucosa with chronic inflammation. A subsequent contrast-enhanced CT scan demonstrated a 4.5×4 cm submucosal mass with focal ulceration in the gastric antrum; this finding was suggestive of GIST. Another 3cm solid and heterogeneously enhancing mass in the pelvic cavity was also detected on the CT scan (Fig. 2). The CEA, CA72-4 and CA19-9 levels were within normal limits; however, the serum CA125 level was elevated to 532.3 U/ml (normal<35 U/ml). We performed 18F-FDG PET/CT for ruling out the malignancy that was suspected due to the abnormal CT findings. 18F-FDG PET/CT showed multiple lesions with intense FDG uptake in the abdominopelvic region (max. SUV; gastric antrum-22.3, retrocaval area-11.4 and pelvic cavity-15.2) (Fig. 3). Although multiplicity of a primary GIST is rare, the presumptive diagnosis was a malignant gastric GIST with metastases or with a synchronous small bowel GIST arising from the distal ileum. She then underwent gastric antrectomy and mass excision. The intraoperative findings included a 5 cm submucosal tumor with central ulceration on the antrum of the stomach. This mass had direct invasion into the head of the pancreas, and the mass was abutted on the transverse colon and mesocolon. A 4 cm hard whitish mass on the right adrenal gland and a 0.5 cm whitish mass on the right subphrenic area were observed. A hydrosalpinx and a 3 cm mutiseptated cyst were found in the pelvic cavity. She underwent gastric antrectomy, mass excision of the right adrenal gland and the right subphrenic area, and right salpingo-oophorectomy.
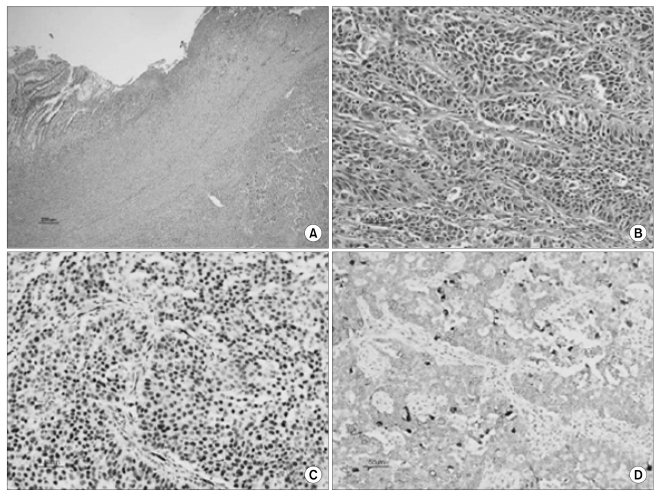
The resected stomach had a protruding tumor that measured 4.5×4×3.5 cm with central ulceration (Fig. 4A). On cut section, this gray-white tumor was situated in the muscularis propria, and it bulged into the subserosa (Fig. 4B). Microscopically, this tumor showed a relatively well-defined border with extensive necrosis. There was a deep ulceration on the overlying mucosa (Fig. 5A). The tumor was composed of irregular sheets of medium-sized cells with nuclear atypia (Fig. 5B). Immunohistochemically, the neoplastic cells were strongly positive for AE1/AE3, cytokeratin 7 and Wilms' tumor-1, whereas they were negative for cytokeratin 20, estrogen receptor and progesterone receptor (Fig. 5C, 5D). The histopathological findings of the gastric tumor were similar to that of the right ovarian serous carcinoma. Based on these findings, the patient underwent a total abdominal hysterectomy, left salpingo-oophorectomy and omentectomy, and peritoneal biopsy was performed, and optimal cytoreduction was achieved. There is no evidence of malignancy in this specimen. These results support the final diagnosis of ovarian serous carcinoma that metastasized to the stomach. The tumor marker checked on postoperative day 7 was CA 125 199.2 U/ml. Her postoperative course was unremarkable and she was discharged on the 9th day following operation and she underwent 6 cycles of chemotherapy with carboplatin and paclitaxel at the outpatient department. One year after therapy, the patient's CA125 level is less than 35 U/ml. She has not experienced a recurrence and she shows no evidence of disease.
DISCUSSION
Epithelial ovarian carcinoma usually metastasizes along the peritoneal surfaces throughout the pelvic cavity and abdominal cavity. The common metastatic sites throughout the abdominal cavity are the omentum, mesentery and serosa of intestine. However, hematogenous dissemination at the time of diagnosis is uncommon, with spread to vital organ parenchyma such as the lung and liver being seen in only about 2% to 3% of these patients. We experienced one case of primary ovarian carcinoma that metastasized to the parenchyma of the stomach. The tumors that most frequently metastasize to the parenchyma of the stomach are reported to be melanoma, lung cancer, breast cancer and other gastrointestinal neoplasm (2). These metastatic lesions are commonly expressed as a submucoal mass with or without central ulceration.
Only one case of intramural stomach metastasis of recurrent epithelial ovarian carcinoma has been reported in English literature (1). But to our best knowledge, there has been no case stomach metastasis of a primary ovarian carcinoma at the time of first diagnosis. In our case, we also didn't consider the possibility of ovarian carcinoma because stomach metastasis of ovarian carcinoma is very rare and the size of the patient's ovarian mass was small (3 cm). So, this patient underwent an operation at the Department of Gastrointestinal Surgery. During the gastric antrectomy and debulking surgery, the general surgeon considered the ovarian mass to be metastatic tumor from the stomach. Morphologically, it was impossible to know the origin of tumor, but immunohistochemical staining indicated the tumor as a primary ovarian origin. Wilms' tumor-1 can arise from both gastric and ovarian origins (3). The presence of ER and PR in epithelial ovarian carcinoma has been known for a long time, but ER and PR are also present in 26% and 20%, respectively, of gastric cancer (4). Importantly, 97% to 100% of non-mucinous ovarian carcinomas are positive for cytokeratin 7, and 70% to 100% are negative for cytokeratin 20 (5-7). In this case, the gastric tissue was positive for cytokeratin 7 and it was negative for cytokeratin 20. This is the first report of intramural gastric metastasis that mimicked GIST from a primary epithelial ovarian carcinoma at the initial disease presentation, and the patient was without a bulky ovarian mass. Although gastric metastasis of ovarian carcinoma is rare, it should be considered in the differential diagnosis of patients that have concurrent stomach and ovarian masses.