AbstractPurposeIn this study, we retrospectively investigated the prevalence of arterioportal (AP) shunts in hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) and evaluated the changes in AP shunts after chemoembolization followed by external beam radiation therapy (EBRT).
Materials and MethodsWe analyzed 54 HCC patients with PVTT who were treated with chemoembolization followed by EBRT. EBRT was uniformly delivered at a total dose of 30 to 45 Gy (median, 35 Gy), with a daily dose of 2 to 4.5 Gy. Angiographic images of chemoembolization before and after radiation therapy (RT) were reviewed to investigate the AP shunt.
ResultsDuring the initial session of chemoembolization, 33 of 54 patients (61%) had an AP shunt. After EBRT, 32 out of 33 patients had an additional session of chemoembolization and were evaluated for a change in the AP shunt. The AP shunt decreased in 20 of 32 patients (63%) after chemoembolization followed by EBRT. The 1-year calculated overall survival (OS) rate for all patients was 52.6% and the 2-year OS was 36.4%. The median OS in all patients was 13 months. Patients with AP shunt showed poorer median OS than those without AP shunt, but there was no statistically significant difference (median, 12 months vs. 17 months).
IntroductionLocally advanced hepatocellular carcinoma (HCC) frequently accompanies portal vein tumor thrombosis (PVTT), with a reported incidence of 21% to 35% [1,2]. Chemoembolization and/or radiation therapy (RT) is a treatment option in Asian-Pacific regions, while sorafenib alone is recommended by the Barcelona Clinic Liver Cancer (BCLC) system for patients with advanced HCC [3,4]. In these patients, an arterioportal (AP) shunt is frequently seen by angiography during chemoembolization. The presence of an AP shunt has been reported in 27% to 63.2% of advanced HCC cases [5,6] and can be caused by PVTT. The sources of AP shunts in HCC with PVTT are the transvasal route through the vasa vasorum, the transplexal route through the peribiliary plexus and the transtumoral route through the draining vein [7,8]. Although many authors have reported the response of PVTT to RT [9,10], there is a lack of data on the changes in AP shunts [11]. In the present study, we investigated the prevalence of AP shunts in HCC patients with PVTT and evaluated the changes in AP shunts after chemoembolization followed by RT.
Materials and Methods1. PatientsIn our institution, patients with unresectable HCC for which chemoembolization alone was expected to be ineffective have been treated with chemoembolization followed by planned external beam RT (EBRT) after a 2-week interval since June 2008. Patients for whom chemoembolization was expected to be ineffective were defined as those patients who had more than one of the following characteristics: tumor size greater than 10 cm, tumor with PVTT, or a previous history of ineffective chemoembolization (twice or more) [12]. Up until December 2010, 204 HCC patients were treated with this protocol. Among them, 54 patients with PVTT who received no prior treatment related to HCC (treatment-naïve) were enrolled in the current study. This research was reviewed and approved by our Institutional Review Board, and informed consent for this research was waived. EBRT was started at a median of 14 days (range, 9 to 21 days) after chemoembolization. PVTT was diagnosed using tri-phasic liver computed tomography (CT), contrastenhanced dynamic magnetic resonance image, or angiography. The presence of PVTT was identified by observation of a low-attenuating intraluminal filling defect adjacent to the primary tumor with arterial enhancement within the thrombus. The location of PVTT was classified into three categories: invading to main portal vein, first order branch (left or right hemi-liver) of the portal vein, and second order branch (segmental) of the portal vein.
2. ChemoembolizationSelective arteriography of the hepatic artery was performed to locate the tumor and any tumor neovascularity. After identifying the tumor-feeding artery, a mixture of adriamycin (Dong-A Pharm, Seoul, Korea) and lipiodol (Guerbet, Aulnay-sous-Bois, France) was slowly injected through the catheter. The adriamycin and lipiodol doses depended on the tumor size and vascularity (3 mg of adriamycin and 1 mL of lipiodol per 1-cm diameter of tumor), though the doses in a single chemoembolization session was limited to 70 mg of adriamycin and 25 mL of lipiodol. When embolization with lipiodol mixture alone was insufficient to block tumor-feeding arteries, additional embolization with 1- to 2-mm in diameter gelatin sponges (Cutanplast, Mascia Brunelli Spa, Milan, Italy) was performed. In patients with an AP shunt, embolization with gelatin sponge particles was initially performed to occlude the shunt. This was followed by infusion of a small amount of lipiodol and adriamycin emulsion. The end point of chemoembolization was complete lipiodol uptake within the tumor or stagnant blood flow in the tumor-feeding arteries by fluoroscopy or injection of the maximum lipiodol dose [13].
3. External beam radiation therapyFour-dimensional CT simulation was performed in all patients. First, a CT scan with contrast-enhancement was performed for the arterial and portal phase during quiet breathing. Four-dimensional images were acquired using the real-time position management system (Varian Medical Systems, Palo Alto, CA) to record the respiratory phase. The respiratory phase was divided into ten equal phases with 0% as end-inspiration and 50% as end-expiration (0% to 90%). The images of 0%, 30%, 50%, 80%, and portal phase were used to delineate targets.
The gross target volumes (GTV), including both the main tumors and PVTT, were delineated at each phase. The main tumor indicates HCC adjacent to PVTT, which invades a portal vein and develops into a tumor thrombus. In several cases (e.g., a large tumor [more than 2/3 of the liver] with severe liver cirrhosis, a huge bilateral intrahepatic tumor with main PVTT, or numerous intrahepatic metastasis), only PVTT was delineated as the GTV. The internal target volume (ITV) was the sum of the GTVs on each phase. A 5-mm margin was added to the ITV to create the planning target volume.
EBRT was delivered at a total dose of 30 to 45 Gy (median, 35 Gy), with a daily dose of 2 to 4.5 Gy using a 6, 10, or 15 MV X-ray. The median number of fractions was 10 (range, 10 to 22). The calculated biologically effective dose as the α/β=10 ranged from 39 to 65.3 Gy10.
4. Evaluation of tumor responseTumor response was assessed by triphasic liver CT at 1 month after completing RT using the modified Response Evaluation Criteria in Solid Tumor (mRECIST) guidelines [14]. A complete response (CR) was defined as a complete disappearance of measurable lesions, a partial response (PR) was defined as a 30% decrease from baseline, progressive disease (PD) was defined as a 20% increase from baseline, and stable disease (SD) was anything between PD and PR. Responding tumors were defined as CR or PR.
5. Evaluation of AP shuntAngiographic images of chemoembolization before and after RT were reviewed by one radiologist to investigate the AP shunt. To analyze the changes in the AP shunt after chemoembolization followed by RT, we developed our own grading system based on angiographic findings that grades AP shunts from 1 to 3. For main PVTT, grade 1 was defined as a shunt flow around the main portal vein (PV) and grade 2 was a shunt flow extending to the superior mesenteric vein or splenic vein. For left or right hemiliver PVTT, grade 1 was defined as a shunt flow around the hemiliver PV, grade 2 was a shunt flow extending to the contralateral hemiliver PV, and grade 3 was a shunt flow extending to the main PV. For segmental PVTT, grade 1 was defined as a shunt flow around the segmental PV, grade 2 was a shunt flow extending to any PV branch above the 1 level, and grade 3 was a shunt flow extending any PV branch above the 2 level. Our own grading system is illustrated in Fig. 1.
6. Statistical analysisThe duration of overall survival (OS) was calculated from the start date of chemoembolization to the date of death or last follow-up. Survival rates were estimated by the Kaplan-Meier method. Survival differences between groups were compared by the log-rank test. The Cox proportional hazards regression model was employed for multivariate analysis. The distribution of categorical variables was analyzed by Fisher exact test. Null hypotheses of no difference were rejected if p-values were less than 0.05, or, equivalently, if the 95% confidence intervals (CIs) of risk point estimates excluded 1. SPSS ver. 19.0 (IBM Co., Armonk, NY) was used for all analyses.
Results1. PatientsFifteen patients (28%) had a main PVTT, 19 (35%) had a left or right hemiliver PVTT, and 20 (37%) had a segmental PVTT. During chemoembolization, embolization with gelatin sponges was performed in 37 patients (69%). RT was administered to both the main tumor and the PVTT in 32 patients (59%), and only to the PVTT in 22 patients (41%). The clinical characteristics of all patients are summarized in Table 1. The median follow-up period was 10 months (range, 2 to 44 months) in all patients.
2. AP shuntDuring the first session of chemoembolization, 33 of 54 patients (61%) had an AP shunt on angiography. After the completion of RT, an additional chemoembolization session was performed in 48 of 54 patients. The median time interval between RT and the second chemoembolization was 31 days (range, 14 to 56 days). Six patients did not have the additional chemoembolization because of death (n=2), loss to follow-up (n=1), development of distant metastases (n=2), and complete remission after the first chemoembolization and RT (n=1). Thus, 48 patients could be evaluated for a change in the grade of the AP shunt after RT using the second angiographic images. The grade changes of AP shunts in these 48 patients are in Table 2. Thirty-two of 48 patients who had an AP shunt initially were analyzed. The grade of AP shunt decreased in 20 of 32 patients (63%) after chemoembolization followed by RT (Fig. 2). The grade of the AP shunt was stable in 11 patients. The AP shunt grade increased from 0 to 1 in one patient. The PVTT response to treatment was associated with the changes in the grade of the AP shunt. The AP shunt grade decreased in 12 of 14 patients (86%) with PVTT response (+) and in eight of 18 patients (44%) with PVTT response (–) (p=0.028). The use of gelatin sponges during chemoembolization did not appear to have any impact on the changes in the AP shunt grade. The AP shunt grade decreased in 13 of 23 patients (57%) who received the embolization with gelatin sponge and in seven of nine patients (78%) who did not (p=0.422).
3. Treatment outcomesOne month after completing RT, tumor response was evaluated. CR was achieved in three patients (6%), PR in 24 (44%), SD in 24 (44%), and PD in three (6%). The 1-year calculated OS rate was 52.6% and the 2-year OS was 36.4%. The median OS was 13 months (95% CI, 7 to 19 months).
Patients with AP shunt have poorer median OS compared to those without AP shunt, but the difference was not statistically significant (median, 17 months [95% CI, 7.2 to 26.8 months] vs. 12 months [95% CI, 7.1 to 16.8]; p=0.186) (Fig. 3). The patients who had decreased AP shunt after treatment showed better median OS than those who did not, but the difference was not statistically significant (median, 12 months [95% CI, 8.2 to 15.8] vs. 8 months [95% CI, 0 to 18.8]; p=0.866).
The presence of lymph node metastasis (p=0.002), multiple tumors (p=0.029), and no tumor response (p=0.001) to treatment were poor prognostic factors for OS. Child class (0.098), tumor size (p=0.201), and the location of PVTT (p=0.270) did not differ significantly. Multivariate analysis showed that tumor response (p=0.020) was the only significant factor for OS.
DiscussionAn AP shunt is frequently associated with advanced HCC. In 1977, Okuda et al. [5] reported that hepatic angiogram revealed various sizes of AP shunts in 63.2% of patients with HCC. A recent report found that of 596 patients with HCC treated with chemoembolization, 27% had a severe AP shunt [15]. An AP shunt associated with HCC may occur by several routes. The draining vein of the tumor vasculature can flow into the portal vein (transtumoral route). The vascular plexus around a large biliary tract (transplexal route) and the vasa vasorum, which originates from a branch of hepatic artery and penetrates the portal vein wall (transvasal route), can also be sources of AP shunts, when portal flow is obstructed or reduced [7,8]. PVTT can cause an AP shunt by both diminishing portal flow and placing tumor vasculature in the thrombus. This study showed that PVTT is frequently associated with an AP shunt (61% of patients).
An AP shunt could be harmful in patients with HCC. Severe AP shunts aggravate the complications of portal hypertension, such as refractory ascites and esophageal varices [16,17], and preclude chemoembolization. The drugs used in chemoembolization pass through the AP shunt and can result in extensive ischemia of normal liver. A severe AP shunt, therefore, needs to be treated. Several authors have reported treatment outcomes of AP shunt embolization [15,18-20]. The long-term effect of ethanol embolization was compared with that of Gelfoam embolization in 97 patients with severe AP shunt [15]. The initial occlusion rate of the AP shunt was 70.3% in the ethanol group and 63.6% in the Gelfoam group, but the recanalization rate at 1 month was 17.8% in the ethanol group and 85.7% in the Gelfoam group. As a result, the complete occlusion rate of AP shunt was 82.8% in the ethanol group and 18.2% in the Gelfoam group after a second treatment.
Only one report has evaluated the effectiveness of RT for an AP shunt [11]. RT (median dose of 60 Gy in 2 or 2.5 Gy per fraction) in 20 patients with arteriovenous shunt (18 of the portal vein and two of the hepatic vein) showed a complete obliteration in four patients and partial obliteration in one patient. As a result, total response of the AP shunt after RT was obtained in five of 20 patients (25%). In the present study, we evaluated the effectiveness of a combination of chemoembolization with RT. Twenty of 32 evaluable patients (63%) had a decrease in the grade of AP shunt and 19 (59%) had complete obliteration (grade 0) of AP shunt. The higher response rate in this study may result from the target volumes for RT in addition to the combined effect with chemoembolization. RT was delivered to PVTT with/without a main tumor and included the shunt area in all patients, while Hsu et al. [11] defined the target volume as the shunt area, and PVTT was included in 13 patients. Because PVTT and the main tumor are the main causes of an AP shunt associated with HCC via the pathways mentioned above, an AP shunt can be reduced or obliterated following RT to the PVTT with/without main tumors by improving portal flow and disrupting the tumor vasculature. This mechanism of obliterating AP shunts associated with HCC by RT may be different in some respects from obliterating cerebral arteriovenous malformations (AVMs) by radiosurgery, in which the vessel is damaged by radiation. Histopathological studies suggested progressive thickening of the intimal layer and thrombosis of the irradiated vessels cause AVM occlusion following radiosurgery. These histopathological changes occur in a few months to years after the irradiation [21]. In the current study, the second chemoembolization was performed at a median of 31 days after RT, so it may be difficult to explain obliteration of AP shunt as the mechanism of AVM occlusion by radiosurgery.
We showed that PVTT response was associated with a decrease in the AP shunt grade. The AP shunt grade decreased in 12 of 14 patients (86%) with PVTT response (+) and in eight of 18 patients (44%) with PVTT response (–) (p=0.028). This finding suggests that a disruption of tumor vasculature and improved portal flow by treatment might partially influence the AP shunt response, but there is a lack of histopathological evidence.
Some authors have reported the prognostic significance of AP shunt changes for OS in HCC patients. Furuse et al. [22] showed that the median survival time in patients with severe AP shunt was worse than in those without severe AP shunt (4.3 months vs. 7.2 months), but the difference was not statistically significant. Hsu et al. [11] reported that patients with complete obliteration after RT had better survival times compared to others (median, 9.2 months vs. 6.3 months; p=0.2808). Huang et al. [15] also found that the complete occlusion rate of the shunt was higher (p < 0.05) and the survival rate was better (p < 0.05) in the group treated with ethanol injection compared to the group treated with Gelfoam. In the current study, patients without AP shunt showed the best median survival time followed by those with decreased AP shunt grade after treatment and those with stable or increased AP shunt grade (17 months, 12 months, and 8 months, respectively), although there was no statistically significant difference.
This study has several limitations. First, the combined effect of chemoembolization and RT for the AP shunt was evaluated. Therefore, this study could not provide any answers about whether RT was independently beneficial in controlling AP shunts and whether the combined effect is better than chemoembolization alone. Second, we could not show the clinical significance of a decrease in AP shunt grade after treatment. Statistically significant differences in OS were not seen in the current study, so a larger scale study might be necessary.
AcknowledgmentsThis research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A2042414). This study was supported by Samsung Medical Center grant [GFO1130081].
Fig. 1.The grading system for arterioportal shunting flow. PVTT, portal vein tumor thrombosis; SMV, superior mesenteric vein; SV, splenic vein; RPV, right portal vein; LPV, left portal vein; arrow, shunt flow; red circle, tumor; orange line, hepatic artery; blue, portal vein. 
Fig. 2.Change in arterioportal (AP) shunt after chemoembolization followed by radiation therapy. (A, B) A 76-year-old patient with left main portal vein tumor thrombus (PVTT) had angiography that showed a grade 2 AP shunt. (A) An AP shunt from the left portal vein (arrow) extends to the right portal vein (arrowhead). (B) After chemoembolization followed by RT, the angiography during the second session of chemoembolization showed minimal shunt flow around the left portal vein (arrow). (C, D) A 56-year-old patient had a S2/4 main tumor and segmental PVTT. (C) The angiography during the first session of chemoembolization showed AP shunt flow to the segmental portal vein (arrows). (D) After chemoembolization followed by RT, the AP shunt flow was completely obliterated. 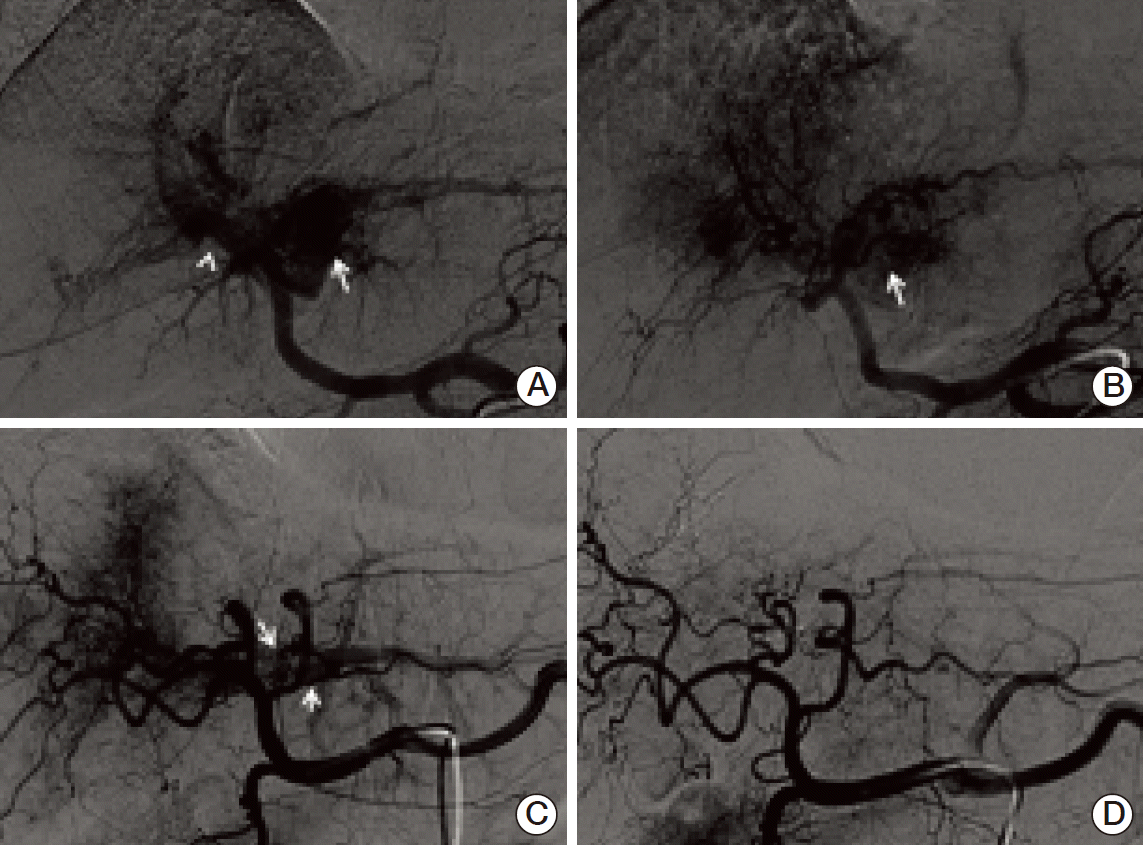
Fig. 3.The overall survival rates by presence of arterioportal (AP) shunt. The median overall survival was 17 months in patients without AP shunt and 12 months in those with AP shunt (p=0.186). 
Table 1.Patient characteristics References1. The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–5.
2. Davidson BR, Gibson M, Dick R, Burroughs A, Rolles K. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation. 1994;57:1174–7.
3. Park HC, Seong J, Tanaka M, Zeng ZC, Lim HY, Guan S, et al. Multidisciplinary management of nonresectable hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:134–40.
4. European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641.
5. Okuda K, Musha H, Yamasaki T, Jinnouchi S, Nagasaki Y, Kubo Y, et al. Angiographic demonstration of intrahepatic arterio-portal anastomoses in hepatocellular carcinoma. Radiology. 1977;122:53–8.
6. Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36–40.
7. Bookstein JJ, Cho KJ, Davis GB, Dail D. Arterioportal communications: observations and hypotheses concerning transsinusoidal and transvasal types. Radiology. 1982;142:581–90.
8. Breen DJ, Rutherford EE, Stedman B, Lee-Elliott C, Hacking CN. Intrahepatic arterioportal shunting and anomalous venous drainage: understanding the CT features in the liver. Eur Radiol. 2004;14:2249–60.
9. Huang YJ, Hsu HC, Wang CY, Wang CJ, Chen HC, Huang EY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1155–63.
10. Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–11.
11. Hsu HC, Chen TY, Chiu KW, Huang EY, Leung SW, Huang YJ, et al. Three-dimensional conformal radiotherapy for the treatment of arteriovenous shunting in patients with hepatocellular carcinoma. Br J Radiol. 2007;80:38–42.
12. Yu JI, Park HC, Lim DH, Kim CJ, Oh D, Yoo BC, et al. Scheduled interval trans-catheter arterial chemoembolization followed by radiation therapy in patients with unresectable hepatocellular carcinoma. J Korean Med Sci. 2012;27:736–43.
13. Oh D, Lim DH, Park HC, Paik SW, Koh KC, Lee JH, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33:370–5.
14. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
15. Huang MS, Lin Q, Jiang ZB, Zhu KS, Guan SH, Li ZR, et al. Comparison of long-term effects between intra-arterially delivered ethanol and Gelfoam for the treatment of severe arterioportal shunt in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:825–9.
16. Ratti F, Cipriani F, Paganelli M, Ferla G, Aldrighetti LA. Surgical approach to multifocal hepatocellular carcinoma with portal vein thrombosis and arterioportal shunt leading to portal hypertension and bleeding: a case report. World J Surg Oncol. 2012;10:34.
17. Nakai M, Sato M, Tanihata H, Sonomura T, Sahara S, Kawai N, et al. Ruptured high flow gastric varices with an intratumoral arterioportal shunt treated with balloonoccluded retrograde transvenous obliteration during temporary balloon occlusion of a hepatic artery. World J Gastroenterol. 2006;12:5404–7.
18. Izaki K, Sugimoto K, Sugimura K, Hirota S. Transcatheter arterial embolization for advanced tumor thrombus with marked arterioportal or arteriovenous shunt complicating hepatocellular carcinoma. Radiat Med. 2004;22:155–62.
19. Murata S, Tajima H, Nakazawa K, Onozawa S, Kumita S, Nomura K. Initial experience of transcatheter arterial chemoembolization during portal vein occlusion for unresectable hepatocellular carcinoma with marked arterioportal shunts. Eur Radiol. 2009;19:2016–23.
20. Sugano S, Miyoshi K, Suzuki T, Kawafune T, Kubota M. Intrahepatic arteriovenous shunting due to hepatocellular carcinoma and cirrhosis, and its change by transcatheter arterial embolization. Am J Gastroenterol. 1994;89:184–8.
|
|
|||||||||||||||||||||||||||||||||||||||||||