AbstractPurposeThis study was conducted to investigate the prognostic value of lymph node ratio (LNR) in stage II/III breast cancer patients who undergo mastectomy after neoadjuvant chemotherapy.
Materials and MethodsClinical and pathological data describing stage II/III breast cancer patients were included in this retrospective study. The primary outcomes were locoregional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS).
ResultsAmong 277 patients, there were 43 ypN0, 64 ypN1, 89 ypN2, and 81 ypN3 cases. Additionally, there were 43, 57, 92 and 85 cases in the LNR 0, 0.01-0.20, 0.21-0.65, and > 0.65 groups, respectively. The median follow-up was 49.5 months. Univariate analysis showed that both ypN stage and LNR were prognostic factors of LRFS, DMFS, DFS, and OS (p < 0.05). Multivariate analysis showed that LNR was an independent prognostic factor of LRFS, DMFS, DFS, and OS (p < 0.05), while ypN stage had no effect on prognosis (p > 0.05).
IntroductionNeoadjuvant chemotherapy is an important component of combination treatment strategies for locally advanced breast cancer. Randomized trials have confirmed that neoadjuvant chemotherapy can achieve the same effects as adjuvant chemotherapy [1], and that tumor response to chemotherapy can be predicted and the breast conservation rate in patients with early-stage breast cancer improved [2-4]. Axillary lymph node metastasis is an important factor in the American Joint Committee on Cancer (AJCC) staging of breast cancer, as well as an important reference indicator that guides the selection of postoperative adjuvant treatment for locally advanced breast cancer. However, due to the varying effects of neoadjuvant chemotherapy on axillary lymph node status in patients with locally advanced breast cancer, the number of axillary lymph nodes detected in postoperative patients who have received neoadjuvant chemotherapy is significantly lower than that in patients who did not receive neoadjuvant chemotherapy [5-9]. This often leads to underestimation of the true axillary lymph node status in these patients, and thus affects prognosis prediction and selection of surgery and adjuvant therapy. Therefore, more appropriate methods for assessment of the status of axillary lymph nodes are needed.
The axillary lymph node ratio (LNR) refers to the ratio of the number of positive axillary lymph nodes to the number of removed lymph nodes at axillary lymph node dissection, and is currently an active field of investigation. Multiple studies have found that LNR is an important prognostic factor in breast cancer patients who have not received neoadjuvant chemotherapy, and its prognostic value may be greater than that of current N staging [10-13]. Investigations of Chinese women with breast cancer have reported similar results [14,15]. However, very few studies have investigated the prognostic value of LNR in patients who received neoadjuvant chemotherapy. Therefore, this study was conducted to evaluate the prognostic values of LNR and ypN surgical staging in stage II/III breast cancer patients who underwent mastectomy after neoadjuvant chemotherapy.
Materials and Methods1. PatientsThis study was performed in accordance with the Declaration of Helsinki and approved by the ethics committee of Sun Yat-Sen University Cancer Center. Written consent was given by the patients for their information to be stored in the hospital database and used for research. Clinical and pathological data describing breast cancer patients who were treated at Sun Yat-Sen University Cancer Center from January 1998 to December 2007 were retrospectively analyzed. Inclusion criteria were (1) female with unilateral breast cancer; (2) clinical stage II/III without distant metastasis at initial diagnosis; (3) received neoadjuvant chemotherapy before surgery; (4) underwent mastectomy and axillary lymph node dissection after neoadjuvant chemotherapy; (5) complete surgical resection of the tumor and negative surgical margins; (6) estrogen receptor (ER), progesterone receptor (PR), human epithelial growth factor receptor family 2 (HER2) status were evaluated; (7) no sign of malignant tumor in other organs at diagnosis.
2. Clinicopathologic factors and lymph node statusClinical, pathological, and immunohistochemical factors including age, menopausal status, initial clinical stage, ypT stage, ypN stage, ER, PR, HER2 status and molecular subtype were used to assess the risk of recurrence and death. ypT and ypN stage were based on the criteria in the 2009 7th edition of the AJCC staging manual for breast cancer. LNR was as defined as in the study by Vinh-Hung et al. [10], and patients were divided into four groups: LNR=0, 0.01-0.20, 0.21-0.65, and > 0.65. More than 1% of the immunostained malignant cells were ER and PR positive. HER2 positivity was indicated by a 3+ or 2+ score upon immunohistochemical evaluation and confirmed using a fluorescence in situ hybridization test for HER2. We could not exactly define breast cancer intrinsic subtypes with immunohistochemistry in all tumors [16] since the exact value of Ki-67 was not available. Therefore, we classified breast cancer intrinsic subtypes as follows: (1) luminal A (ER+ and/or PR+, HER2–); (2) luminal B (ER+ and/or PR+, HER2+); HER2 enriched (ER−, PR– and HER2+); and (3) triple negative (ER−, PR– and HER2–) [17].
3. Follow-up and survival endpointsAfter initial diagnosis, patients were followed up once every 3-6 months. The endpoints of the study were locoregional recurrence-free survival (LRFS), distant metastasisfree survival (DMFS), disease-free survival (DFS), and overall survival (OS). Locoregional recurrence was defined as a pathologically proven recurrence in the ipsilateral chest wall, supraclavicular and infraclavicular areas, axilla, and internal mammary region. Distant metastasis was defined as recurrence at any site other than those defined as locoregional recurrence, which was confirmed by imaging studies and pathological examination of a tissue specimen when necessary. OS was defined as breast cancer and non-breast cancer related deaths.
4. Statistical analysisThe Kaplan-Meier method was used to calculate survival rate and plot the survival curve, and differences were examined using the log-rank test. A Cox regression model was performed using the stepwise method, and significant variables in univariate analysis (p < 0.05) were entered into a multivariate Cox regression model. SPSS ver. 16.0 (SPSS Inc., Chicago, IL) was used for all analyses, and a value of p < 0.05 was considered to indicate statistical significance.
Results1. General informationA total of 277 patients were included in this study. The median age at diagnosis was 47 years (range, 27 to 74 years), and the preoperative clinical stage was stage II in 30 cases (10.8%) and stage III in 247 cases (89.2%). The median number of lymph nodes removed at axillary lymph node dissection was 16 (range, 3 to 47), and the median number of positive lymph nodes was 5 (range, 0 to 46). The ypN stage was ypN0 in 43 cases (15.5%), ypN1 in 64 cases (23.1%), ypN2 in 89 cases (32.2%), and ypN3 in 81 cases (29.2%). There were 43 cases in the LNR 0.0 group (15.5%), 57 in the 0.01-0.20 group (20.6%), 92 in the 0.21-0.65 group (33.2%), and 85 in the > 0.65 group (30.7%). The clinical and pathological factors of the patients are summarized in Table 1.
2. Treatment and responseOverall, 259 patients (93.5%) received neoadjuvant chemotherapy with an anthracycline or taxane regimen, while 18 (6.5%) received a cyclophosphamide, methotrexate, and 5-fluorouracil regimen. The median number of chemotherapy courses was three (range, 2 to 8). After neoadjuvant chemotherapy, 35 patients (12.6%) achieved a pathologic complete response of breast tumor and axillary lymph nodes.
All patients underwent mastectomy and axillary lymph node dissection after neoadjuvant chemotherapy. Following surgery, 265 patients received adjuvant chemotherapy (95.7%), 255 of whom received an anthracycline or taxane regimen. Radiotherapy was performed in the ipsilateral chest wall and supraclavicular and infraclavicular lymph drainage area in 156 patients (54.2%). Patients with positive hormone receptors received endocrine therapy. Premenopausal patients were treated with tamoxifen, and postmenopausal patients were treated with tamoxifen or an aromatase inhibitor. None of the patients with HER2+ cancers received trastuzumab.
3. Survival and disease progressionThe median follow-up duration was 49.5 months (range, 6 to 144 months), during which time 53 patients (19.1%) experienced locoregional recurrence, 134 (48.4%) experienced distant metastases, and 105 (37.9%) died of breast cancer. In addition, two patients died of cardiovascular disease. The overall 5-year and 10-year LRFS were 78.4% and 70.9%, the 5-year and 10-year DMFS were 51.7% and 38.9%, the 5-year and 10-year DFS were 50.0% and 37.0%, and the 5-year and 10-year OS were 62.0% and 51.7%, respectively.
4. Univariate and multivariate analysesThe results of univariate analysis of clinicopathologic factors and prognostic factors are shown in Table 1. Both ypN stage and LNR were significant prognostic factors of LRFS, DMFS, DFS, and OS. The Kaplan-Meier survival curves of ypN and LNR are shown in Fig. 1. Statistically significant variables in the univariate survival analysis were entered into the multivariate Cox proportional hazards model (Table 2). The LNR and ypN were input into the model simultaneously as covariates. The results showed that LNR remained a significant independent prognostic factor of LRFS, DMFS, DFS, and OS (p < 0.05), while ypN was no longer a significant prognostic factor (p > 0.05).
DiscussionRetrospective analysis of 277 Chinese female patients who underwent total mastectomy after neoadjuvant therapy revealed that higher LNR was associated with a poorer prognosis.
Previous investigations of the prognostic value of LNR in breast cancer have focused on patients who did not receive neoadjuvant chemotherapy, while few studies of patients who received neoadjuvant chemotherapy have been conducted. Indeed, there have been only two comprehensive studies of the prognostic value of LNR in breast cancer patients who received neoadjuvant chemotherapy to date. Keam et al. [5] analyzed 205 patients who received docetaxel and doxorubicin-based neoadjuvant chemotherapy and found a LNR > 0.25 to be an independent prognostic factor of relapse-free survival and OS, which was superior to that of the ypN stage. Saxena et al. [18] studied 314 breast cancer patients who received neoadjuvant chemotherapy using the same LNR grouping method employed in our study and found that LNR was an independent prognostic factor of survival, and its prognostic value was poorer than that of ypN stage. Our results showed that the prognostic value of LNR was significantly better than that of ypN stage. Although findings regarding whether the prognostic value of LNR is superior to that of ypN stage differed, all study findings suggest that LNR is an important prognostic factor.
In the current AJCC staging system for breast cancer, the pN stage is based on the number of positive axillary lymph nodes. However, it has been reported that, when compared with breast cancer patients who did not receive neoadjuvant chemotherapy, more patients who received neoadjuvant chemotherapy had less than 10 axillary lymph nodes removed at dissection [8,9]. Therefore, it is believed that neoadjuvant chemotherapy may affect the actual lymph node status, and is not conducive to estimation of prognosis and guidance of subsequent adjuvant therapy.
Although the value of axillary lymph node status prior to neoadjuvant chemotherapy for estimating prognosis and guiding subsequent adjuvant therapy remains controversial in breast cancer patients [19], our results indicate that LNR can reflect axillary lymph node status in post-neoadjuvant chemotherapy breast cancer patients more accurately than pN stage. Veronesi et al. [20] suggested that pN stage be accompanied by the LNR to accurately reflect lymph node status, and that clinicians should have a more intuitive understanding of the LNR. Moreover, the use of the LNR may allow the effects of variation in lymph node dissection level among surgeons to be reduced [21].
Different studies have adopted various LNR cutoff points for grouping [10,21-23]. Vinh-Hung et al. [10] reported that the cutoff points of 0.20 and 0.65 have better prognostic value than pN stage in breast cancer patients who did not receive neoadjuvant chemotherapy. The present study also revealed that the prognostic value of these LNR cutoff values for grouping was better than that of yN stage. The International Nodal Ratio Working Group is investigating the prognostic value of LNR in breast cancer [23,24]. Hopefully, their work can confirm the prognostic value of LNR in breast cancer and provide a consensus regarding the optimal LNR cutoff values.
It should be noted that this study had a number of limitations. First, it was a single-center retrospective study; however, the results confirm the growing body of literature that supports the prognostic value of LNR in breast cancer. Second, patients with HER2 positivity exceeded 40% in present study due to selection bias, and the results of this study suggest that HER2 significantly affected the prognosis of patients, but patients in this study did not undergo trastuzumab therapy and it is unclear whether targeted therapy would change these findings. Finally, LNR was evaluated after a median of three cycles of chemotherapy in the present study, and most patients received adjuvant chemotherapy. It is uncertain whether LNR after three cycles of chemotherapy or more is useful.
AcknowledgmentsThis study was supported by a grant from the Sci-Tech Office of Guangdong Province (No. 2008B060600019), the Youth Foundation of the First Affiliated Hospital of Xiamen University (No. XYY2012005) and the Education Scientific Research Foundation of Young Teachers of Fujian Province (No. JB13131).
Fig. 1.Comparison of Kaplan-Meier curves for different lymph node ratios (LNRs) and ypN stages. (A, E) Locoregional recurrence-free survival. (B, F) Distant metastasis-free survival. (C, G) Disease-free survival. (D, H) Overall survival. pN (A-D) and LNR (E-H). 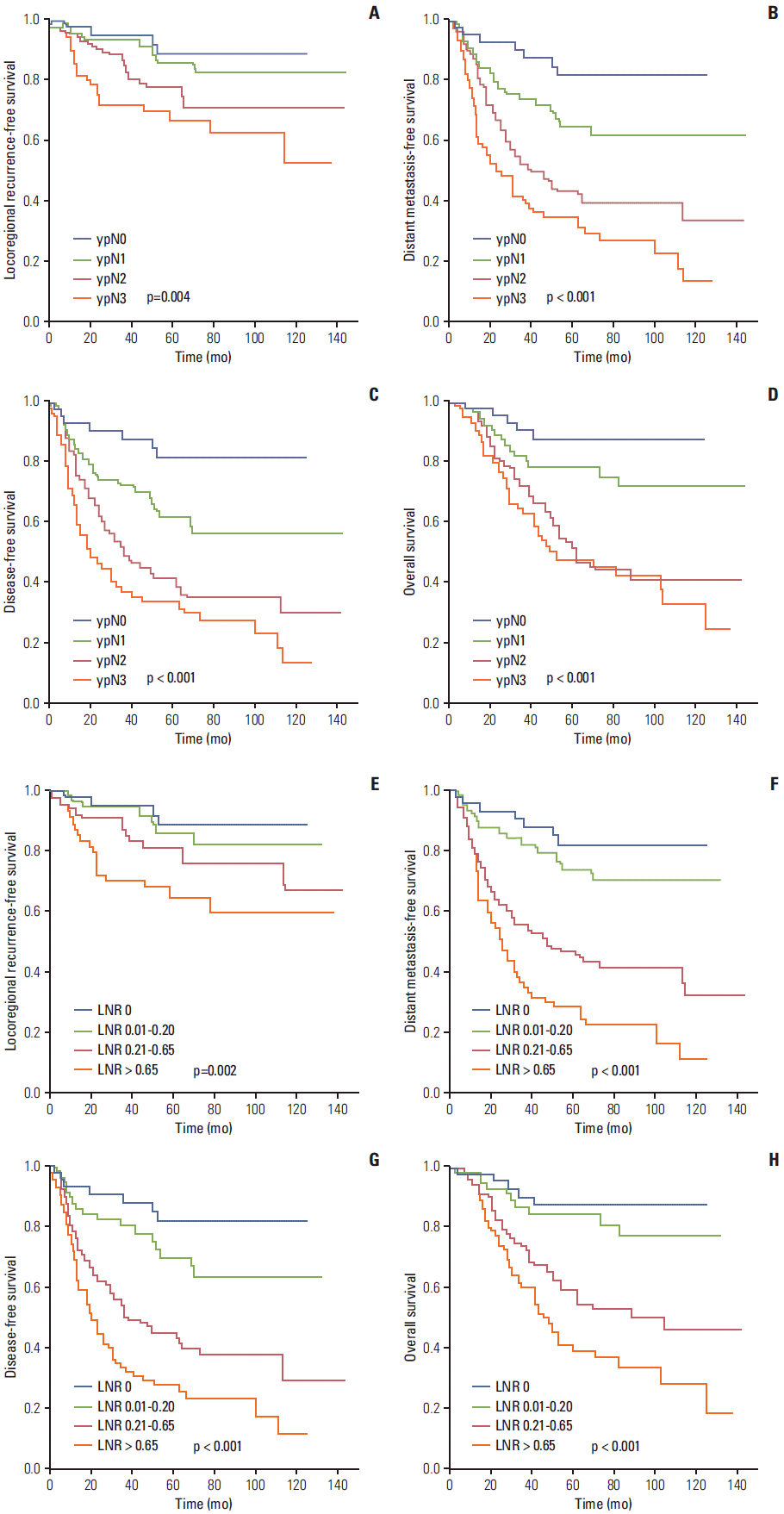
Table 1.Patient characteristics and univariate survival analyses of prognostic factors
Table 2.Multivariate analysis of survival
References1. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94.
2. Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21:2600–8.
3. Estevez LG, Gradishar WJ. Evidence-based use of neoadjuvant taxane in operable and inoperable breast cancer. Clin Cancer Res. 2004;10:3249–61.
4. Shimizu C, Ando M, Kouno T, Katsumata N, Fujiwara Y. Current trends and controversies over pre-operative chemotherapy for women with operable breast cancer. Jpn J Clin Oncol. 2007;37:1–8.
5. Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009;116:153–60.
6. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
7. Baslaim MM, Al Malik OA, Al-Sobhi SS, Ibrahim E, Ezzat A, Ajarim D, et al. Decreased axillary lymph node retrieval in patients after neoadjuvant chemotherapy. Am J Surg. 2002;184:299–301.
8. Belanger J, Soucy G, Sideris L, Leblanc G, Drolet P, Mitchell A, et al. Neoadjuvant chemotherapy in invasive breast cancer results in a lower axillary lymph node count. J Am Coll Surg. 2008;206:704–8.
9. Neuman H, Carey LA, Ollila DW, Livasy C, Calvo BF, Meyer AA, et al. Axillary lymph node count is lower after neoadjuvant chemotherapy. Am J Surg. 2006;191:827–9.
10. Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–8.
11. Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA Jr. Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1,788 patients with long-term follow-up. J Am Coll Surg. 2010;210:797–805.
12. Chagpar AB, Camp RL, Rimm DL. Lymph node ratio should be considered for incorporation into staging for breast cancer. Ann Surg Oncol. 2011;18:3143–8.
13. Ahn SH, Kim HJ, Lee JW, Gong GY, Noh DY, Yang JH, et al. Lymph node ratio and pN staging in patients with node-positive breast cancer: a report from the Korean breast cancer society. Breast Cancer Res Treat. 2011;130:507–15.
14. Wu SG, Chen Y, Sun JY, Li FY, Lin Q, Lin HX, et al. Using the lymph nodal ratio to predict the risk of locoregional recurrence in lymph node-positive breast cancer patients treated with mastectomy without radiation therapy. Radiat Oncol. 2013;8:119.
15. Wu SG, He ZY, Li Q, Sun JY, Li FY, Lin Q, et al. Prognostic value of metastatic axillary lymph node ratio for Chinese breast cancer patients. PLoS One. 2013;8:e61410
16. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
17. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–54.
18. Saxena N, Hartman M, Aziz R, Rapiti E, Bhoo Pathy N, Lim SE, et al. Prognostic value of axillary lymph node status after neoadjuvant chemotherapy: Results from a multicentre study. Eur J Cancer. 2011;47:1186–92.
19. Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, et al. Staging of breast cancer in the neoadjuvant setting. Cancer Res. 2008;68:6477–81.
20. Veronesi U, Zurrida S, Viale G, Galimberti V, Arnone P, Nole F. Rethinking TNM: a breast cancer classification to guide to treatment and facilitate research. Breast J. 2009;15:291–5.
21. Truong PT, Woodward WA, Thames HD, Ragaz J, Olivotto IA, Buchholz TA. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1-3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2007;68:59–65.
22. Castrucci W, Lannin D, Haffty BG, Higgins SA, Moran MS. Using nodal ratios to predict risk of regional recurrences in patients treated with breast conservation therapy with 4 or more positive lymph nodes. ISRN Surg. 2011;2011:874814.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||