AbstractPurposeThe aim of this study is to determine the diagnostic and prognostic role of baseline spinal magnetic resonance imaging (MRI) in patients with multiple myeloma.
Materials and MethodsWe enrolled patients newly diagnosed with multiple myeloma from 2004-2011 at a single center. Abnormal MRI findings that were not detected in radiographs have been analyzed and categorized as malignant compression fractures or extramedullary plasmacytoma. The bone marrow (BM) infiltration patterns on MRI have been classified into five categories.
ResultsA total of 113 patients with a median age of 65 years (range, 40 to 89 years) were enrolled in the study. Malignant compression fractures not detected in the bone survey were found in 26 patients (23.0%), including three patients (2.6%) with no related symptoms or signs. Extramedullary plasmacytoma was detected in 22 patients (19.5%), including 15 (13.3%) with epidural extension of the tumor. Of these 22 patients, 11 (50.0%) had no relevant symptoms or signs. The presence of malignant compression fractures did not influence overall survival; whereas non-epidural extramedullary plasmacytoma was associated with poor overall survival in the multivariate analysis (hazard ratio, 3.205; 95% confidence interval [CI], 1.430 to 9.845; p=0.042). During the follow-up for a median of 21 months (range, 1 to 91 months), overall survival with the mixed BM infiltrative pattern (median, 24.0 months; 95% CI, 22.9 to 25.1 months) was shorter than those with other patterns (median 56 months; 95% CI, 48.9 to 63.1 months; p=0.030).
IntroductionMultiple myeloma is a neoplastic plasma cell disorder characterized by clonal proliferation of malignant plasma cells in the bone marrow (BM) microenvironment, leading to bone destruction and marrow failure [1]. The initial diagnostic workup in all patients with multiple myeloma includes a full skeleton X-ray survey to evaluate the lytic bone lesions. For detection of occult lesions, magnetic resonance imaging (MRI) of the spine and pelvis is mandatory in all patients with a presumed diagnosis of smoldering multiple myeloma [2,3]. In case of symptomatic multiple myeloma, several guidelines recommend MRI only in certain circumstances, such as ambiguous plain radiographic findings or suspected cord compression, and for delineating the nature and extent of soft tissue masses [2,4]. On the contrary, MRI has been considered as a part of routine evaluation since unsuspected focal lesions and soft tissue plasmacytoma involving the spine and pelvis can be visualized and patterns of MRI abnormality may have prognostic significance [3,5,6]. Thus, the role of a baseline MRI in patients with symptomatic multiple myeloma, who have no related symptoms or signs, remains controversial. In this study, we evaluated the diagnostic and prognostic implications of spinal MRI in patients who were newly diagnosed with multiple myeloma.
Materials and Methods1. Patients and baseline evaluationWe retrospectively analyzed all consecutive patients who were newly diagnosed with symptomatic multiple myeloma and underwent a whole-spine MRI before initiation of antimyeloma therapy from 2004-2011 at Chungnam National University Hospital (Daejeon, Korea). Multiple myeloma work-up included serum and urine protein electrophoresis, quantitation of serum immunoglobulin levels, 24-hour urinary protein excretion, serum β2 microglobulin and BM aspirations and biopsies for a morphologic interpretation. Plain X-ray skeletal surveys were performed with digital radiographs, including posterior-anterior chest, a skull series, lateral view of the vertebral column, and anterior-posterior views of the pelvis, shoulders, and extremities. We classified the patients using the Durie-Salmon and international staging systems (ISS). The study protocol was approved by the local institutional review board.
2. MRI evaluationA series of MRI sequences were performed to permit identification of the BM infiltration pattern and soft tissue masses, including sagittal fat-suppressed T2-weighted magnetic resonance (MR) images, sagittal T1-weighted MR images, axial T1- and T2-weighted MR images, and Gd-enhanced axial and sagittal T1-weighted MR images of the whole spine. MRI was analyzed based on a consensus by two radiologists.
MRI abnormalities not detected in digital radiographs were classified into two categories: malignant compression fractures and extramedullary plasmacytoma. Malignant compression fractures were defined as compression fractures with a contour bulging of the involved masses. Extramedullary plasmacytoma was classified into two categories, epidural extension and others, such as plasmacytoma in the rib or pelvis (Fig. 1).
Additionally, the patterns of BM infiltration were classified into the following five categories: homogenously diffuse infiltrative, micronodular, macronodular, mixed, and normal appearance (Fig. 2).
3. Statistical analysisA Kaplan-Meier analysis was used to estimate overall survival with group comparisons completed using a log-rank test. Overall survival was defined from the date of diagnosis until death from any cause, and survivors were censored at the time of last contact. Univariate and multivariate analyses of prognostic factors were performed using a Cox regression analysis. We evaluated the correlation between the two factors, such as MRI abnormalities and ISS, using a χ2 test. Basic statistical data were obtained using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL). Statistical significance is represented by two-tailed p-values, with a cut-off value of 0.05.
Results1. Patients’ characteristicsA total of 113 patients with a median age of 65 years (range, 40 to 89 years) were enrolled in the study, and the male to female ratio was similar. The median follow-up duration was 21 months (range, 1 to 90.5 months). Based on the Durie-Salmon staging system, 61.1% of patients were stage IIIA and 22.1% of patients were stage IIIB; whereas based on the ISS, 40.7% were stage III, followed by 38.9% in stage II (Table 1).
2. MRI abnormalitiesMalignant compression fractures were observed in 26 patients (23%), and epidural extensions of plamacytoma were observed in 15 patients (13.3%), while non-epidural extensions of plamacytoma were observed in seven (6.2%). Malignant compression fracture combined with epidural extension was observed in six patients and non-epidural extension of plasmacytoma in two patients. Two patients had both epidural and non-epidural extensions of plasmacytoma. In total, 38 patients (33.6%) had MRI abnormalities not shown in digital radiographs. Of the patients with malignant compression fracture, three (11.5%) had no related symptoms or signs. Of the patients with epidural and non-epidural extensions, seven (46.7%) and four (57.1%), respectively, had no related symptoms or signs. Malignant compression fracture and epidural extension of plasmacytoma was significantly related to the symptoms and signs, but non-epidural extension of plasmacytoma did not correlate to any of the related symptoms and signs (Table 2). There was no significant correlation between MRI abnormalities and ISS (Table 3). Malignant compression fracture did not affect the overall survival (p=0.056); however, patients with extramedullary plasmacytoma had significantly poorer survival rates (p < 0.001) with either epidural extension (p=0.009) or nonepidural extension (p=0.028) of plasmacytoma (Fig. 3).
3. Patterns of BM infiltration observed with MRIDiffuse infiltrative pattern was found in 35 patients (31.0%), mixed pattern in 17 (15.0%) and normal pattern in seven (6.2%). Patterns of BM infiltration did not correlate with ISS (Table 4). However, all of the patients with normal pattern had < 33% BM plasma cells (Table 5). Patterns of BM infiltration did not influence overall survival (p=0.244) (Fig. 4A). However, patients with mixed BM infiltrative patterns tended to have poorer overall survival. Thus, when patients were divided into two groups, mixed patterns showed significantly poorer survival rates than the other group (p=0.030) (Fig. 4B). Epidural extension and non-epidural extension of the plasmacytoma, ISS, creatinine, total calcium, and lactate dehydrogenase were statistically significant in an univariate analysis. In a multivariate analysis using these prognostic factors, non-epidural extramedullary plasmacytoma and ISS III were significant prognostic indicators of poor outcome (hazard ratio [HR], 3.49; 95% confidence interval [CI], 1.126 to 10.819; p=0.03 and HR, 2.722; 95% CI, 1.109 to 6.681; p=0.029, respectively) (Table 6).
DiscussionA skeletal survey in patients with multiple myeloma was performed conventionally by a plain whole body X-ray [2,3]. However, plain X-rays had limitations since some areas were not well visualized or had low sensitivity, and analysis was observer-dependent [7]. Although the usefulness of novel imaging modalities, such as computed tomography (CT) or positron emission tomography (PET), has been reported, MRI remains the standard imaging technique for detecting spine or pelvic lesions and for evaluating patterns of BM infiltration [8,9]. For example, Baur-Melnyk et al. [10], reported that a whole-body, multi-detector CT led to a significantly lower detection rate than a whole-body MRI in patients with multiple myeloma. Furthermore, Zamagni et al. [11] and Fonti et al. [12], demonstrated that although PET-CT provides additional and valuable information for the assessment of myeloma bone disease, MRI is superior with regard to the assessment of BM involvement in the spine and pelvis.
In our study, a whole-spine MRI could detect additional skeletal abnormalities that are not shown on digital radiographs in approximately 33% of patients. Moreover, extramedullary plasmacytoma in these abnormalities is correlated with poor prognosis. Since some patients did not present any related symptoms and signs, we suggest that a whole-spine MRI is needed as a baseline skeletal survey in all patients. In addition, baseline whole-spine MRI has prognostic implications in the detection of focal lesions and patterns of BM infiltration. Walker et al. [13], reported that MRI was a more powerful tool for detecting focal lesions than plain X-ray bone surveys since 52% of patients with normal metastatic bone survey had a focal lesion on MRI, leading to the conclusion that MRI can be routinely used for staging, prognosis, and response assessment in myeloma. The percentage of patients in the aforementioned report was higher than our current study because they included osteolytic lesions as a focal lesion.
We classified patterns of BM infiltration in MRI into five categories based on a previous description [14]. In the literature, a micronodular pattern was described as a “salt-and-pepper” pattern, a macronodular pattern as a focal infiltrative pattern, and a mixed pattern as a combined or variegated pattern [5,7]. The prognostic significance of diffuse and focal infiltration patterns has been demonstrated previously, whereby patients with a diffuse pattern of infiltration had features of more advanced disease and poorer overall survival [6,15-17]. In addition, Walker et al. [13], demonstrated that the number of focal lesions had independent prognostic implications. In our study, we did not find a correlation between infiltrative patterns and ISS, and similar to the previous report, mixed patterns resulted in a poorer prognosis since they have both focal lesions and diffuse infiltration. It is clear that a large number of focal lesions or extents of involved lesions affect the prognosis. Recently, dynamic contrast-enhanced MRI was used to evaluate the prognosis of multiple myeloma, suggesting that functional MRI techniques may in the near future for identifying patients who could benefit most from anti-angiogenic drugs [18].
Serial MRI monitoring can be useful in assessing the response to treatment [19]. In our study, we did not perform a follow-up MRI in all patients with anti-myeloma therapy; thus, we could not evaluate the changes in MRI patterns or infiltration, although the data warrant a prospective study of serial MRI monitoring.
There was little selection bias in our study since we performed the whole-spine MRI consecutively in all patients diagnosed with multiple myeloma from the beginning of enrollment in 2004 and treated patients using the same strategy at a single institution.
ConclusionIn summary, this study demonstrates the importance of a baseline spinal MRI in patients with multiple myeloma. Spinal MRI revealed bone lesions, as well as extramedullary plasmacytoma, which may not be detected in plain radiographs, even in patients with no relevant symptoms or signs. Furthermore, mixed infiltrative patterns observed with MRI predicted poor overall survival. We conclude that spine MRI at the time of diagnosis is beneficial not only to surveying skeletal lesions, but also assessing the prognosis in patients with multiple myeloma.
Fig. 1.(A) Malignant compression fracture, which was defined as a collapsed body with contour bulging of the involved masses. Extra-medullary extension of plasmacytoma, including epidural extension (B, indicated by an arrow) and non-epidural extension (C, indicated by arrows). 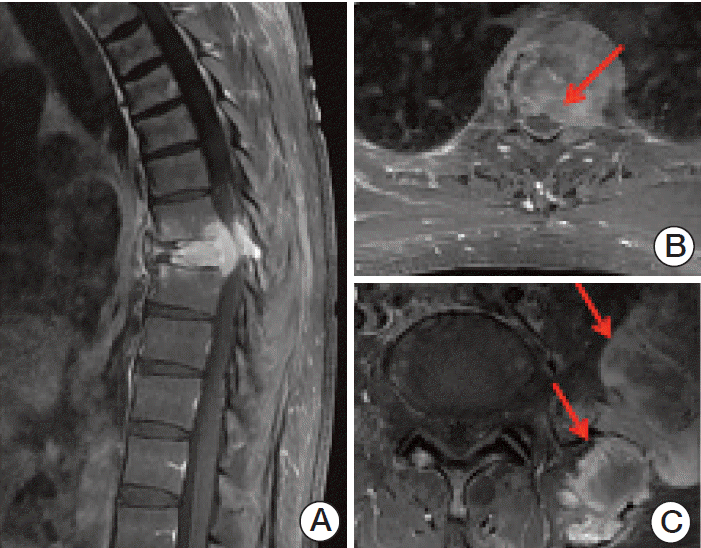
Fig. 2.(A) Diffuse infiltrative pattern. Homogeneous hypointense change which is similar or lower than disc signal on T1-weighted image. (B) Micronodular pattern, also referred to as “salt-and-pepper” pattern. Small foci of low intensity throughout the marrow on sagittal T1-weighted image. (C) Macronodular pattern. Multifocal relatively large hypointense lesions are detected on sagittal T1-weighted image. (D) Mixed pattern. It shows a combined micronodular and macronodular lesions. (E) Normal bone marrow pattern. Bone marrow shows hyperintense compared to the disc on T1-weighted image. 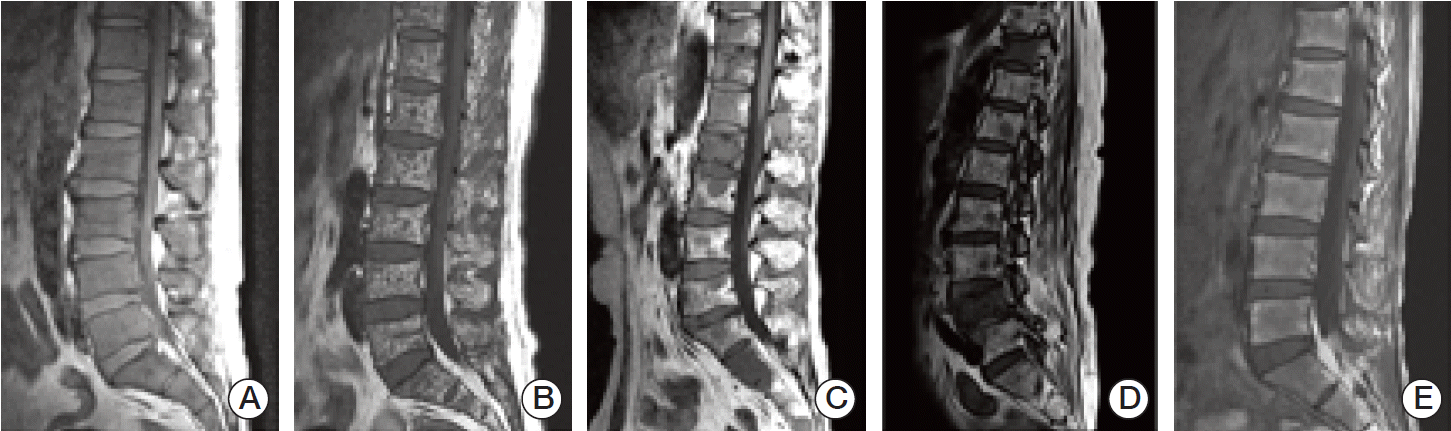
Fig. 3.Kaplan-Meier plots of survival according to magnetic resonance imaging abnormalities. (A) Survival according to malignant compression fracture. (B) Survival according to extramedullary extension of plasmacytoma. (C) Survival according to epidural extension of plasmacytoma. (D) Survival according to non-epidural extension of plasmacytoma. 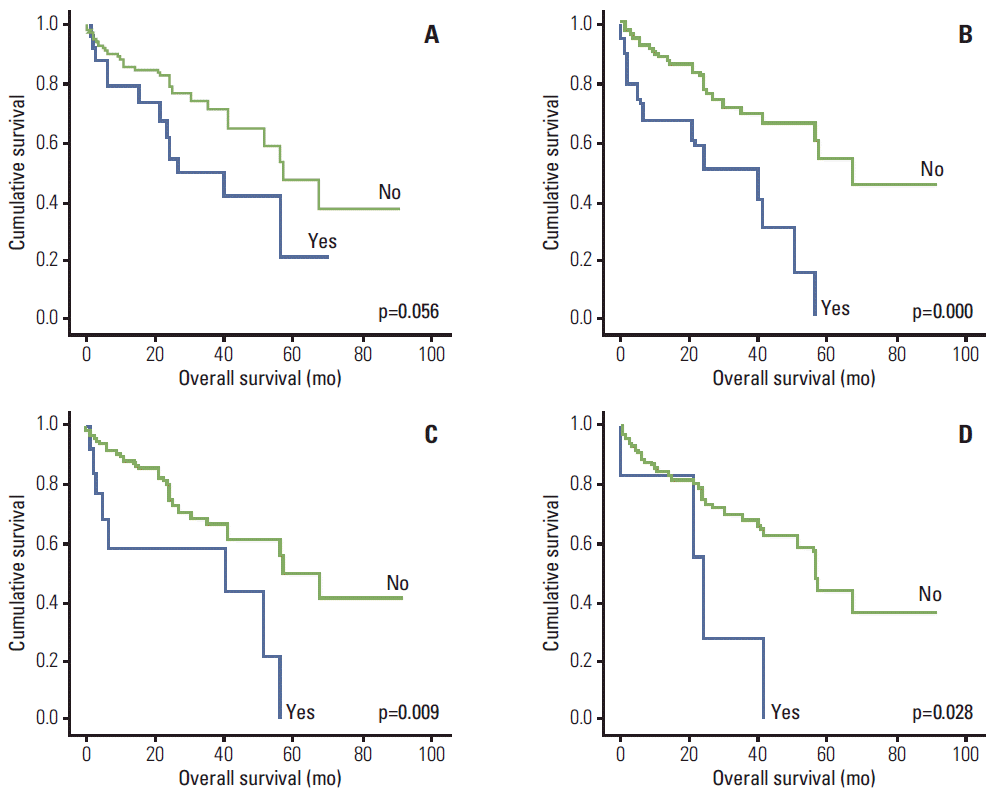
Fig. 4.Kaplan-Meier plots of survival according to bone marrow infiltrative patterns. (A) Survival according to each of bone marrow infiltrative patterns. (B) Survival according to mixed patterns and others. 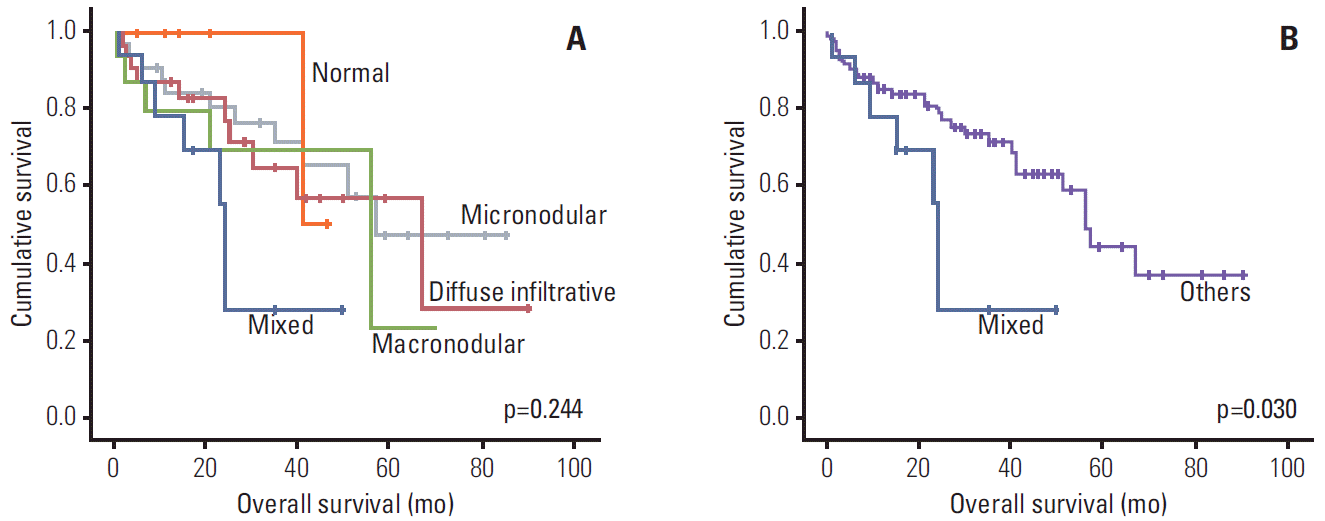
Table 1.Patients’ baseline characteristics (n=113) Table 2.Correlation of MRI abnormalities and related symptoms/signs (n=113)
Table 3.Correlation of MRI abnormalities and international staging system (n=113)
Table 4.Correlation of MRI infiltrative patterns and international staging system (n=113)
Table 5.Correlation of BM infiltrative patterns and percentage of plasma cells (n=113)
Table 6.Comparison of univariate and multivariate correlates of survival duration
References2. Bird JM, Owen RG, D'Sa S, Snowden JA, Pratt G, Ashcroft J, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75.
3. Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–5.
4. D'Sa S, Abildgaard N, Tighe J, Shaw P, Hall-Craggs M. Guidelines for the use of imaging in the management of myeloma. Br J Haematol. 2007;137:49–63.
5. Baur-Melnyk A, Buhmann S, Durr HR, Reiser M. Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol. 2005;55:56–63.
6. Moulopoulos LA, Gika D, Anagnostopoulos A, Delasalle K, Weber D, Alexanian R, et al. Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol. 2005;16:1824–8.
7. Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23:1545–56.
8. Hillengass J, Landgren O. Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging "early myeloma". Leuk Lymphoma. 2013;54:1355–63.
9. Tan E, Weiss BM, Mena E, Korde N, Choyke PL, Landgren O. Current and future imaging modalities for multiple myeloma and its precursor states. Leuk Lymphoma. 2011;52:1630–40.
10. Baur-Melnyk A, Buhmann S, Becker C, Schoenberg SO, Lang N, Bartl R, et al. Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am J Roentgenol. 2008;190:1097–104.
11. Zamagni E, Nanni C, Patriarca F, Englaro E, Castellucci P, Geatti O, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–5.
12. Fonti R, Salvatore B, Quarantelli M, Sirignano C, Segreto S, Petruzziello F, et al. 18F-FDG PET/CT, 99mTc-MIBI, and MRI in evaluation of patients with multiple myeloma. J Nucl Med. 2008;49:195–200.
13. Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25:1121–8.
14. Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010;30:127–42.
15. Stabler A, Baur A, Bartl R, Munker R, Lamerz R, Reiser MF. Contrast enhancement and quantitative signal analysis in MR imaging of multiple myeloma: assessment of focal and diffuse growth patterns in marrow correlated with biopsies and survival rates. AJR Am J Roentgenol. 1996;167:1029–36.
16. Kusumoto S, Jinnai I, Itoh K, Kawai N, Sakata T, Matsuda A, et al. Magnetic resonance imaging patterns in patients with multiple myeloma. Br J Haematol. 1997;99:649–55.
17. Baur A, Stabler A, Nagel D, Lamerz R, Bartl R, Hiller E, et al. Magnetic resonance imaging as a supplement for the clinical staging system of Durie and Salmon? Cancer. 2002;95:1334–45.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||