AbstractPurposeIn this study, we investigated the clinical characteristics and treatment results of osteosarcoma during the past 7 years, and evaluated the role of high dose chemotherapy (HDCT) with autologous stem cell transplantation (ASCT).
Materials and MethodsWe retrospectively analyzed the clinical data of patients who were diagnosed as osteosarcoma at our center from January, 2000 to December, 2007.
ResultsThe 5-year overall survival and event-free survival of the patients were 72.6% and 55.9%, respectively. Seventeen (41.5%) patients showed disease progression during treatment or relapse after the end of treatment. The patients who had metastasis at diagnosis or who had a lower grade of necrosis after neoadjuvant chemotherapy showed decreased overall and event-free survival. Four patients received ASCT after HDCT, and 3 of them are alive without disease.
INTRODUCTIONOsteosarcoma is the most common primary bone tumor in childhood and adolescence. It occurs preferentially in male, and develops primarily during the puberty undergoing rapid growth. In the past, when surgery was the only treatment, most patients showed relapse after amputation, and the 5-year survival rate was less than 10% (1). With the introduction of neoadjuvant chemotherapy, the 5-year survival increased up to 70% and limb-salvage operation became possible improving the quality of life (2-5). However, treatment outcomes are still poor in cases of relapse or refractory osteosarcoma or cases showing metastasis at the time of diagnosis.
We analyzed the clinical characteristics, prognostic factors, and treatment outcomes of patients treated for osteosarcoma, and evaluated the role of autologous hematopoietic stem cell transplantation (ASCT) following high dose chemotherapy (HDCT) to improve the treatment outcome of osteosarcoma.
MATERIALS AND METHODSWe retrospectively analyzed the medical record of 41 patients who were diagnosed and treated as osteosarcoma at Seoul National University Children's Hospital from January, 2000 to December, 2007.
For diagnosis, simple radiography and magnetic resonance imaging (MRI) were taken on the primary site, and chest radiography, chest computed tomography (CT) and bone scan were taken to assess metastasis. Biopsy of primary site was done for pathologic diagnosis.
Neoadjuvant chemotherapy was administered to all cases except 3 cases which were suspected to be benign tumors such as chondroma or meningioma and thus surgical resections were performed. Neoadjuvant chemotherapy was administered by two methods largely depending on whether intra-arterial chemotherapy was feasible or not. For patients for whom intra-arterial chemotherapy was feasible with primary sites at the distal femur or the proximal tibia, 130 mg/m2 cisplatin was administered for 2 hours through an intra-arterial catheter, and 60 mg/m2 doxorubicin was infused continuously for 72 hours intravenously, which was repeated 4 times at 3 weeks interval. For cases with tumors in the site where the intra-arterial approach was not possible or intra-arterial chemotherapy was no longer feasible because of lesions in the skin or muscles, chemotherapy was administered through the vein. In such cases, cisplatin (120 mg/m2, d0), doxorubicin (25 mg/m2, d1-3), and high dose methotrexate (12 g/m2, d21, d28) were administered two times at 5 weeks interval. After the neoadjuvant chemotherapy, for cases that prosthesis required for surgery was not ready, chemotherapy was administered one cycle more than the planned cycle. Depending on the clinical features after neoadjuvant chemotherapy, limb salvage operation or amputation was conducted. As postoperative adjuvant chemotherapy, cisplatin (120 mg/m2, d0), doxorubicin (25 mg/m2, d1-3), and high dose methotrexate (12 g/m2, d21, d28) were administered 4 times at 5 weeks interval, or cisplatin (120 mg/m2, d0) and ifosfamide (1,800 mg/m2, d0-4) were administered alternately at 5 weeks interval. For some patients, chemotherapy using bleomycin, cyclophosphamide, and dactinomycin was administered additionally 3~6 times. Chest radiography was taken in each cycle to assess cardiomegaly and echocardiography was done when the accumulated dose of doxorubicin exceeded 100 mg/m2, 200 mg/m2 or 300 mg/m2. For cases showing the impairment of cardiac function, chemotherapy was switched to the regimen containing ifosfamide, carboplatin, and etoposide.
For recurred patients, cyclophosphamide, topotecan, etoposide, and high dose methotrexate were used primarily, and surgical treatment for the recurred area was carried out before or after the chemotherapy. From 2006, ASCT with HDCT were performed in patients recurred or patients with pulmonary metastasis at diagnosis.
In all patients, during the treatment, simple radiography on the primary site and chest CT were taken to assess the disease progression at 3 months interval. Survival time was obtained based on the date of diagnosis, and event was defined as disease progression, relapse, or death. Kaplan-Meier analysis and log-rank univariate comparisons were used to evaluate overall survival and event-free survival.
RESULTS1) Clinical characteristicsDuring the study period, 42 patients were diagnosed as osteosarcoma. Among them, one patient was diagnosed as periosteal osteosarcoma and thus excluded from the analysis, and total 41 patients were analyzed. The clinical characteristics of patients were summarized in Table 1. The median age of patients was 11.5 years (range: 6~15 years), and 28 patients (68.3%) were male. Distal femur was the most common primary site with 22 cases (53.7%). Nine patients (22.0%) had pulmonary metastasis at the time of diagnosis and two patients (4.9%) had bone metastasis. Osteoblastic type was the most prevalent pathologic type with 30 cases (73.2%), chondroblastic type was observed in 7 cases (17.1%), and in 1 patient, giant cell-rich osteosarcoma which is rare variant was observed. Pathologic subtypes were not classifiable in 3 patients because tissues obtained by biopsy prior to chemotherapy were not sufficient for the classification and tissues after surgery showed extensive necrosis in 2 patients and severe tissue deformity in 1 patient.
Total 38 patients received neoadjuvant chemotherapy, and 3 patients were suspected to have benign tumors initially and thus tumor resections were carried out without neoadjuvant chemotherapy. Among the 38 patients, 21 patients received intra-arterial cisplatin. Two patients developed dermatitis and myositis after receiving intra-arterial chemotherapy, hence, chemotherapy was switched to intravenous injection. Amputations were carried out on 3 patients, and 2 of them underwent amputation at a later time after limb salvage operation because of poor blood circulation or non-union.
The degree of necrosis level is shown in Table 1. The proportion of patients who showed more than 90% necrosis was higher in the intra-arterial chemotherapy group (66.7%) than in the intravenous group (40%), but the difference was not significant (p=0.175).
2) Recurrent cases or patients with disease progression during treatmentsSeventeen patients (41.5%) showed disease progression during the treatments or relapse after the completion of treatments (Table 2). Excluding 3 patients whose follow up observations were ceased, 9 patients of 14 patients (64.3%) died because of the progression of disease, and 1 of them showed recurrence of disease after ASCT. Among the remaining 5 patients, 3 patients are alive without disease after receiving ASCT, 1 patient is alive after pulmonary metastectomy and secondary chemotherapy, and the remaining 1 patient is currently under secondary chemotherapy.
3) ASCT following HDCTThe HDCT followed by ASCT was performed on 4 patients (Table 3). The ASCT was done because of relapse in 2 patients, disease progression in 1 patient and metastasis at diagnosis in 1 patient. Three patients received melphalan (140 mg/m2, d-7 and 70 mg/m2, d-6), etoposide (200 mg/m2, d-8~d-5), and carboplatin (400 mg/m2, d-8~d-5) as HDCT, and 1 patient received melphalan (140 mg/m2, d-7 and 70 mg/m2, d-6), topotecan (2 mg/m2, d-8~d-4), and carboplatin (500 mg/m2, d-5~d-3). During the HDCT, one patient (No. 16) showed acute renal failure with 5.8 mg/dL of serum creatinine level and 15.24 ml/min/m2 of glomerular filtration rate, but the patient improved after supportive treatment without dialysis. Other severe side effects such as veno-occlusive disease or sepsis were not shown. In all four patients, transplants engrafted well, and 3 patients are under the follow up observation without the evidence of recurrence. In one patient, the disease recurred 3 months after ASCT and the patient died because of the disease progression.
4) SurvivalThe follow up duration of the entire patients was average 46 months (4~98 months). The 5-year overall survival was 72.6% and event-free survival was 55.9%. The 5-year overall survival and event-free survival of the group treated with intraarterial chemotherapy were 82.0% and 63.8%, respectively, which were higher than the survival rates of the intravenous chemotherapy group (63.5% and 50.2%, respectively), but the differences were not significant (p=0.208, p=0.385). The 5-year overall survival (43.8%) and event-free survival (11.1%) of patients with metastasis at diagnosis were lower than the overall survival (80.0%) and event free survival (69.7%) of the patients without metastasis (p=0.014 and p=0.000) (Fig. 1). Also, the 5-year overall survival (51.6%) and event-free survival (29.2%) of patients who showed necrosis less than 90% after the neoadjuvant chemotherapy were lower than the overall survival (94.7%) and event free survival (84.4%) of the patients whose necrosis rates were over 90% (p=0.012 and p=0.002) (Fig. 2).
DISCUSSIONThe use of chemotherapy has significantly changed the treatment outcome of osteosarcoma. The long term survival rate was less than 20% when patients received surgery only (1), but the survival rate reached 50~70% after the introduction of chemotherapy in the 1970s (2-5). Particularly, by the administration of neoadjuvant chemotherapy, limb salvage surgery became feasible, and the response to neoadjuvant chemotherapy was able to be used as a good marker to predict the long term survival rate (6,7). Drugs that are shown to be effective on osteosarcoma are cisplatin, methotrexate, doxorubicin and ifosfamide, and present chemotherapy is based on these four drugs (8,9). However, established treatment protocols are absent for recurrent cases or refractory osteosarcoma unresponsive to conventional therapies, and various clinical trials are ongoing.
In our study, the 5-year survival rate of the entire patients did not differ from the survival rate reported previously. But the rate of recurrence or progression during the treatment with conventional drugs was 41.5%, and the mortality rate was high in these patients. In addition, the long term prognosis was poor in patients with metastasis at the time of diagnosis and patients poorly responded to neoadjuvant chemotherapy. Therefore, for the improvement of the treatment outcome of osteosarcoma, alternative therapeutic options are required for relapsed patients, unresponsive patients, patients with metastasis at the time of diagnosis, and patients with poor response to neoadjuvant chemotherapy.
We performed ASCT following HDCT from the year of 2006 for patients with recurred osteosarcoma or patients with metastasis at the time of diagnosis. Until now, 4 patients received ASCT, and 3 of them are alive without disease. Two of them are alive with long term disease-free survival of longer than 2 years and 6 months. For osteosarcoma, ASCT following HDCT has been attempted by several groups previously, and it has been reported that many patients showed disease remission after HDCT and their survival time was prolonged (10,11). However, one study reported that patients who showed remission after ASCT relapsed ultimately in many cases, and thus the 3-year survival rate was approximately 20% (10). We performed HDCT using melphalan, carboplatin, and etoposide (or topotecan). Although the follow up duration is not sufficient yet, based on the present results, ASCT with HDCT could be an alternative treatment option in recurrent patients, refractory patients, patients with metastasis at the time of diagnosis and patients poorly responded to neoadjuvant chemotherapy. Long term follow ups on a larger number of patients are required in the future.
We administered intra-arterial cisplatin in cases intra-arterial chemotherapy was feasible. From the 1990s, intra-arterial chemotherapy with cisplatin has been attempted, and the effect on the long term survival rate is controversial in comparison with the intravenous administration. But, the tumor necrosis rate was found to be superior to the intravenous injection (12,13). It has been also reported that the treatment could be done readily, and the treatment response was also good (14). In our study, although it was not statistically significant, the necrosis rate and survival rate were higher in the intra-arterial chemotherapy group compared to the intravenous group. Therefore, it is required to analyze the long term prognosis of the intra-arterial administration of cisplatin in a larger number of patients.
CONCLUSIONWith the introduction of chemotherapy, treatment outcome of osteosarcoma showed a remarkable improvement. However, the long term survival rate is still low in the recurrent patients, refractory patients, patients with metastasis at the time of diagnosis, and patients with poor response to neoadjuvant chemotherapy. Therefore, to improve the treatment outcome of osteosarcoma, more aggressive treatments for such patients are required, and HDCT with ASCT could be one of the alternative approaches.
NotesThis study was supported by a grant of the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0620460-1). References1. Marcove RC, Mike V, Hajek JV, Levin AG, Hutter RV. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970;52:411–423. PMID: 5269156
2. Bacci G, Picci P, Ferrari S, Ruggieri P, Casadei R, Tienghi A, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993;72:3227–3238. PMID: 8242546
3. Bacci G, Picci P, Ruggieri P, Mercuri M, Avella M, Capanna R, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Istituto Rizzoli Experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990;65:2539–2553. PMID: 2337871
4. Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. PMID: 9667263
5. Saeter G, Alvegard TA, Elomaa I, Stenwig AE, Holmstrom T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9:1766–1775. PMID: 1717666
6. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. PMID: 11821461
7. Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer. 1992;69:698–708. PMID: 1730120
8. Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19:341–346. PMID: 17545797
9. Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. PMID: 16860938
10. Fagioli F, Aglietta M, Tienghi A, Ferrari S, Brach del Prever A, Vassallo E, et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. J Clin Oncol. 2002;20:2150–2156. PMID: 11956277
11. Kasper B, Lehnert T, Bernd L, Mechtersheimer G, Goldschmidt H, Ho AD, et al. High-dose chemotherapy with autologous peripheral blood stem cell transplantation for bone and soft-tissue sarcomas. Bone Marrow Transplant. 2004;34:37–41. PMID: 15170176
12. Bielack SS, Bieling P, Erttmann R, Winkler K. Intraarterial chemotherapy for osteosarcoma: does the result really justify the effort? Cancer Treat Res. 1993;62:85–92. PMID: 8096763
13. Jaffe N, Prudich J, Knapp J, Wang YM, Bowman R, Cangir A, et al. Treatment of primary osteosarcoma with intra-arterial and intravenous high-dose methotrexate. J Clin Oncol. 1983;1:428–431. PMID: 6607977
14. Choi HS, Kang HJ, Lee JA, Han HJ, Park HJ, Seong KW, et al. Treatment Result of Pediatric Osteosarcoma with Intraarterial Cisplatin. J Korean Cancer Assoc. 1998;30:169–177.
Fig. 1The 5-year overall survival (43.8%) and event-free survival (11.1%) of patients with metastasis at diagnosis were lower than the overall survival (80.0%) and event free survival (69.7%) of the other patients (p=0.014 and p=0.000). 
Fig. 2The 5-year overall survival (51.6%) and event-free survival (29.2%) of patients who showed necrosis less than 90% after the neoadjuvant chemotherapy were lower than the overall survival (94.7%) and event free survival (84.4%) of the other patients (p=0.012 and p=0.002). 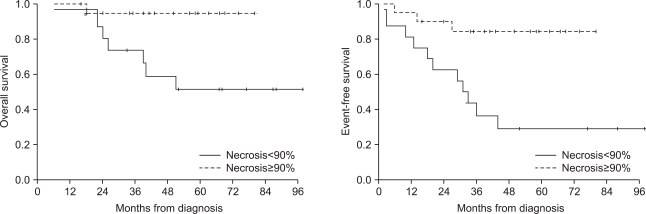
Table 2Characteristics of patients who showed relapse or disease progression during treatment  *intraarterial cisplatin with intravenous doxorubicin, †intravenous cisplatin with intravenous doxorubicin, ‡is consisted of cisplatin, doxorubicin and high-dose methotrexate, §is consisted of cisplatin, doxorubicin, ifosfamide and high-dose methotrexate, ∥no evidence of disease. CCG: Children's Oncology Group. |
|
|||||||||||||||||||||||||||||||||||||||||