AbstractPurposeThe purpose of this study was to evaluate the role of immediate postoperative radiotherapy (RT) in adult patients with a low-grade oligodendroglioma (LODG).
Materials and MethodsA total of 74 patients, older than 15 years, were treated in our institution between April 1990 and March 2006 for newly diagnosed LODGs. After surgery, 43 patients were treated with immediate RT with a total dose of 54~55.8 Gy with 1.8 Gy fractions (RT group) and 31 patients were followed with no adjuvant RT (OP group). All patients were closely observed until tumor progression or death with frequent work-ups including neurological examinations and MRI. Primary endpoints were overall survival and progression-free survival. The median follow-up duration of survivors was 6.2 years in the RT group and 5.8 years in the OP group.
ResultsMedian progression-free survival was 13.2 years in the RT group and 4.6 years in the OP group; multivariate analysis confirmed improved outcome with the use of immediate RT (hazard ratio, 0.22; 95% confidence interval-CI, 0.09~0.55; p<0.001). Median overall survival was 14.9 years in the RT group and 9.8 years in the OP group; the use of adjuvant RT was also associated with a trend toward better overall survival after immediate RT based on multivariate analysis (hazard ratio, 0.3; 95% CI, 0.08~1.17; p=0.082). No severe RT related complications were observed.
IntroductionOligodendrogliomas are the second most common form of gliomas that are encountered in adults. The proportion of oligodendrogliomas may represent as many as 2% to 4% of primary brain tumors and 25 to 33% of glial tumors (1-4). Low-grade oligodendrogliomas (LODGs), World Health Organization grade 2 tumors, represent 2.7% of glial tumors (5). As oligodendrogliomas are infiltrative and poorly demarcated from the surrounding neural tissue, total resection is often not feasible and effective adjuvant treatment might be required. Although many treatment recommendations have been based on studies for low-grade gliomas (6-8) where the use of an appropriate dose of radiotherapy (RT) could prolong progression-free survival but not overall survival, the optimal management of LODGs remains controversial.
The objectives of this study were to evaluate long-term results and to analyze the benefit of immediate operative RT for the improvement of outcome of patients with an LODG.
Materials and Methods1. PatientsWe retrospectively reviewed 74 consecutive patients older than 15 years with a previously untreated LOGD at a supratentorial location. Ineligibility criteria were the presence of an infratentorial or disseminated disease (including gliomatosis cerebri), treatment at another institution and a previous diagnosis of other cancers. Between April 1990 and March 2006, the diagnosis of LODG according to the WHO Classification was confirmed in 105 patients; 23 patients were younger than 16 years, six patients had infratentorial or disseminated disease and two patients were excluded from the analysis due to lack of completion of radiotherapy and early follow-up loss. The investigational review board of the hospital approved the clinical medical records review and the study protocol.
An initial clinical work-up was performed with a physical and neurological examination and all patients underwent a baseline MRI scan. All patients underwent a biopsy or tumor removal and diagnoses were confirmed after pathological examinations. The Karnofsky Performance Scale was used to evaluate the postoperative performance status. The extent of resection was mainly based on the description of the surgeon, as postoperative images were not available for all cases. The extent of resection was categorized as a biopsy, partial removal (less than 50% of tumor removal), subtotal removal (50~95% of tumor removal) and gross total removal (more than 95% of tumor removal).
2. TreatmentOf the 74 eligible patients, 31 patients were treated with surgery alone (OP group) and 43 patients were treated with the use of immediate radiotherapy (RT group). In the OP group, two patients underwent a biopsy and two patients underwent partial removal of the tumor. The patients were closely observed until progression and were salvaged with RT or surgical removal of tumors. In the RT group, there were 10 patients diagnosed with the use of a biopsy alone and one patient underwent partial removal of the tumor. Only two patients received first-line chemotherapy (procarbazine, lomustine and vincrisine-PCV) and all of the patients underwent RT.
3. RadiotherapyRadiotherapy was administered five days a week using a continuous course of treatment with photons produced with a megavoltage linear accelerator. Most patients (86%) started radiotherapy within six weeks after a histological diagnosis. The total radiation dose ranged from 54 to 55.8 Gy, with a median dose of 54 Gy using a conventional fractionation schedule of 1.8 Gy per fraction per day. RT fields encompassed the postoperative tumor volume and tumor bed (as defined by a CT or MRI scan) with a 2-cm margin. Depending on the tumor location and size, patients were treated with different techniques, such as two parallel-opposed fields, two angled fields with a wedge filter or multiple convergent fields.
4. Statistical analysisThe endpoints of this study were overall survival and progression-free survival. The follow-up period and time to death or progression were measured from the date of surgical diagnosis. If there was any evidence of tumor growth as determined on the imaging work-ups, tumor growth was defined as tumor progression without regard to the development of symptoms or signs. Survival rates were calculated using the Kaplan-Meier method. The log-rank test was used for univariate analysis, and the Cox proportional hazard model was used for multivariate analysis. All statistically significant prognostic variables by the use of univariate analysis were included in the multivariate analysis. Statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL) and differences were considered statistically significant for p<0.05 (two-sided test).
Results1. Patient and tumor characteristicsPatient and tumor characteristics are shown in Table 1. At baseline, the OP and RT group patients did not differ with regard to the Karnofsky performance status, seizure development, tumor size and tumor enhancement. Of the 74 patients, 42 patients were men and 32 patients were women. The differences in the male to female ratio and the median age between the OP and RT group patients were statistically significant. In the RT group, there were more males (p=0.002) and older (p=0.028) patients. Tumors occupying the frontal lobe were more common (p=0.05) and gross tumor removal was performed more frequently (p=0.003) in the OP group.
2. Survival and prognostic factorsSurvival at five years and 10 years from the time of diagnosis were 88% and 43% for the OP group, respectively, and 95% and 73% for the RT group, respectively. Median survival was 9.8 years (95% confidence interval-CI, 5.4~14.2 years) in the OP group versus 14.9 years (95% CI, 12.6~17.1 years) in the RT group; no difference in survival was observed with the use of the log-rank test (p=0.141) (Fig. 1). However, patients in the RT group had a longer period of progression-free survival as compared to patients in the OP group (p=0.004) (Fig. 2). Median progression-free survival was 4.6 years (95% CI, 3.2~6.0 years) in the OP group versus 13.2 years (95% CI, 7.5~18.9 years) in the RT group.
Univariate analysis of all patient-related and tumor-related variables determined that tumor size and extent of tumor removal were significant prognostic factors for overall survival (Table 2). Based on multivariate analysis using a model with five factors that were entered for a significance level of p<0.15, improved survival was significantly associated with a good performance status (p=0.018), smaller tumor size (p=0.03) and gross tumor removal (p=0.003); however, patients undergoing immediate RT had a marginal significance (p=0.082). With regard to progression-free survival, the use of univariate analysis confirmed that an initial presentation of seizure, tumor size and undergoing immediate RT had prognostic significance (Table 3). Multivariate analysis was performed using five factors; four factors were entered in the model for a significance level of p<0.15 and the extent of tumor removal was considered to have an impact on progression-free survival. Gross tumor removal (p=0.035) and undergoing immediate RT (p=0.001) were confirmed as independent prognostic factors for progression-free survival.
3. Salvage treatmentDisease failure occurred in 15 patients after RT and most patients (n=13) were salvaged with the use of an aggressive modality including re-irradiation in 10 patients, chemotherapy in six patients and surgery in six patients. The chemotherapy regimen included procarbazine, lomustine and vincrisine (PCV) in most patients unless the agents had been previously administered. In the OP group, 15 patients experienced failure and 14 patients were treated with additional modalities, including RT in 11 patients, chemotherapy in eight patients and surgery in seven patients. The response rate after salvage was 38% in the RT group and 64% in the OP group.
4. Complications of RTIn general, immediate RT was tolerable in patients. Acute minor complications were noted during RT or within six months after RT. The most commonly reported toxicities were nausea and vomiting (26%), anorexia (16%), headache (14%) and otitis (9%). Five patients presented with long-term sequelae that interfered with daily life activities, including two patients with radiation demyelination. Severe radiation toxicity, defined as grade 3 to grade 5 central nervous system (CNS) toxicity, was not observed during RT or after RT.
DiscussionThis study has shown that immediate RT in adult patients with a supratentorial LODG increased the median progression-free survival by nine years, without severe CNS sequelae. Moreover, there was a trend towards better overall survival with the use of immediate RT. The absence of an overall survival benefit for the use of immediate RT is probably due to the effectiveness of salvage RT. For tumor progression, 11 (73%) of 15 patients who had been treated with surgery alone at the time of initial presentation received salvage RT.
Overall survival at five years after RT was 95% and five-year progression-free survival was 81%. We believe our results are comparable to the best results that have been previously reported. Five-year overall survival rates have ranged from 51% to 89%, and 10-year survival rates have ranged from 29% to 85% in a series of LOGDs treated with surgery and radiotherapy (4,9-12). One possible explanation for the high overall survival rates in our study is that aggressive salvage treatments were performed in most patients with recurrence in our study. Treatment results and the salvage rate in our series are similar to findings reported from the Memorial Sloan Kettering Cancer Center (12), where the best reported overall survival rate has been demonstrated. In that study, recurrence was diagnosed in 72 patients (68%) and treatment utilized at the time of first recurrence was chemotherapy in 32 patients, radiochemotherapy in 16 patients, radiotherapy in 13 patients and surgery alone in nine patients; most patients received PCV chemotherapy.
In our study, all patients had undergone MR imaging at the time of the initial presentation. As MR imaging is the most sensitive test available to diagnose brain tumors, the use of MRI is probably the investigation of choice. Moreover, the RT field was determined using an MR image; enhancing lesions and suspicious abnormalities as seen on T2-weighted images or fluid attenuated inversion recovery (FLAIR) images were considered as tumors. As tumor cells of an oligodendroglioma are likely to extend beyond MR-defined abnormalities (13), the use of MR for gross tumor volume delineation and addition of an adequate margin probably guaranteed more accurate targeting of tumors as compared to the use of CT based treatment. For the monitoring of brain tumors, in the same manner, it is crucial to identify progression or treatment failure early during follow-up to change treatment schemes and thereby to optimize patient outcome. Frequent use of MR during follow-up in this study probably helped to provide optimal salvage treatment.
An LODG is a radio-responsive tumor and it can be assumed that immediate RT in patients with an LODG is beneficial when added to surgery. However, a good radiation response may not be translated into an advantage in overall survival. The series studied by Bauman et al. demonstrated no statistically significant association between RT response and progression-free survival of patients with a low-grade glioma (14). More importantly, in the milestone EORTC 22845 randomized trial, immediate RT after surgery prolonged progressionfree survival but did not affect overall survival (7). Indeed, RT was usually performed after surgery in LODGs as a standard treatment, but evidence for efficacy of RT for LODGs has not been well established as for low-grade gliomas. In some studies, an overall survival advantage has been shown with RT after surgery (15) or for patients who had undergone subtotal resection (16,17). In our study, there were more males, older patients and more residual tumors in the RT group as compared to the OP group. With regard to these worse factors, a definite benefit in progression-free survival and marginally better overall survival seemed to provide some evidence of the efficacy of the use of immediate RT.
The most frequently identified prognostic factors for LODGs are age (18), tumor size (12), performance status (19), hemiparesis (4) and the use of postoperative RT (20). Yeh et al. have suggested that a benefit for postoperative RT exists regardless of the extent of surgical resection (21). These investigators reported better progression-free and overall survival with RT based on the use of multivariate analysis. It is noteworthy that all (n=52) patients in that study had supratentorial LODGs without exception. The use of immediate RT was associated with better overall and progression-free survival in the present study. Multivariate analysis also showed a significant correlation between the use of immediate RT and better progression-free survival; marginal significance for overall survival was demonstrated. The most powerful factor to predict overall survival was the extent of surgery.
Severe or fatal CNS toxicities were not observed during or after RT in this study, perhaps due to the low daily-administered dose and the total dose. As shown in prospective trials, the incidence of radionecrosis has been reported below 2% with moderate doses of RT ranging from 45 Gy to 54 Gy with the use of 1.8 Gy daily fractions. Late neurocognitive defects of patients after RT of the CNS are well documented. The EORTC trial that compared low versus high dose RT in low-grade gliomas reported an analysis of quality of life (22). Patients treated with a dose of 59.4 Gy were reported as having a lower functional level as compared with a dose of 45 Gy. Recently, a large sized survey on cognitive deficits in progression-free survivors of low-grade gliomas failed to confirm an association between RT and cognitive deficits (23). Only a fraction size greater than 2 Gy and treatment with anti-epileptic drugs was associated with cognitive dysfunction in patients.
Deferring RT until recurrence or progression is a viable option in patients with a good prognosis after cytoreductive surgery. This situation delays any potential side effects of radiation until treatment is clearly necessary. A report from the University of Florida has suggested that deferred treatment (surgery and/or RT) until symptomatic progression does not compromise outcome for selected patients with oligodendrogliomas (24). Favorable overall survival in patients with deferred-treatment was predictable for patients who were diagnosed at a young age, presented with seizures alone and for patients with good performance status in that study, although the number of patients was insufficient to provide a conclusive recommendation. In addition, many current ongoing treatment protocols follow a treatment strategy that shows a clear trend toward treatment delay, deferring intervention until tumor progression. However, fear that RT increases CNS toxicity is controversial; as demonstrated in our study, the potential benefit of immediate RT should be considered when RT is indicated for patients with LODGs.
ConclusionOur findings suggest that immediate RT is beneficial for patients with a supratentorial LODG for the improvement of progression-free survival and overall survival without severe RT-induced adverse effects. Immediate postoperative RT can therefore be implemented as an effective and safe modality in LDOG therapy. However, to define clearly the potential benefit for overall survival, randomized studies are needed in the future.
References1. Mork SJ, Lindegaard KF, Halvorsen TB, Lehmann EH, Solgaard T, Hatlevoll R, et al. Oligodendroglioma: incidence and biological behavior in a defined population. J Neurosurg. 1985;63:881–889. PMID: 4056902
2. Daumas-Duport C, Varlet P, Tucker ML, Beuvon F, Cervera P, Chodkiewicz JP. Oligodendrogliomas. Part I: Patterns of growth, histological diagnosis, clinical and imaging correlations: a study of 153 cases. J Neurooncol. 1997;34:37–59. PMID: 9210052
3. Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. PMID: 9083161
4. Gannett DE, Wisbeck WM, Silbergeld DL, Berger MS. The role of postoperative irradiation in the treatment of oligodendroglioma. Int J Radiat Oncol Biol Phys. 1994;30:567–573. PMID: 7928487
5. CBTRUSPrimary brain tumors in the united states. Statistical Report 1997-2001. 2004. Hinsdale: CBTRUS.
6. Karim AB, Maat B, Hatlevoll R, Menten J, Rutten EH, Thomas DG, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–556. PMID: 8948338
7. van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. PMID: 16168780
8. Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. PMID: 11980997
9. Berrino F. The EUROCARE Study: strengths, limitations and perspectives of population-based, comparative survival studies. Ann Oncol. 2003;14(Suppl 5):S9–S13.
10. Combs SE, Schulz-Ertner D, Thilmann C, Edler L, Debus J. Fractionated stereotactic radiation therapy in the management of primary oligodendroglioma and oligoastrocytoma. Int J Radiat Oncol Biol Phys. 2005;62:797–802. PMID: 15936562
11. Nijjar TS, Simpson WJ, Gadalla T, McCartney M. Oligodendroglioma. The Princess Margaret Hospital experience (1958-1984). Cancer. 1993;71:4002–4006. PMID: 8508366
12. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54:1442–1448. PMID: 10751254
13. Daumas-Duport C, Scheithauer BW, Kelly PJ. A histologic and cytologic method for the spatial definition of gliomas. Mayo Clin Proc. 1987;62:435–449. PMID: 2437411
14. Bauman G, Pahapill P, Macdonald D, Fisher B, Leighton C, Cairncross G. Low grade glioma: a measuring radiographic response to radiotherapy. Can J Neurol Sci. 1999;26:18–22. PMID: 10068802
15. Wallner KE, Gonzales M, Sheline GE. Treatment of oligodendrogliomas with or without postoperative irradiation. J Neurosurg. 1988;68:684–688. PMID: 3357029
16. Lindegaard KF, Mork SJ, Eide GE, Halvorsen TB, Hatlevoll R, Solgaard T, et al. Statistical analysis of clinicopathological features, radiotherapy, and survival in 170 cases of oligodendroglioma. J Neurosurg. 1987;67:224–230. PMID: 3598683
17. Shimizu KT, Tran LM, Mark RJ, Selch MT. Management of oligodendrogliomas. Radiology. 1993;186:569–572. PMID: 8421767
18. Schiffer D, Dutto A, Cavalla P, Bosone I, Chiò A, Villani R, et al. Prognostic factors in oligodendroglioma. Can J Neurol Sci. 1997;24:313–319. PMID: 9398978
19. Allam A, Radwi A, El Weshi A, Hassounah M. Oligodendroglioma: an analysis of prognostic factors and treatment results. Am J Clin Oncol. 2000;23:170–175. PMID: 10776979
20. Celli P, Nofrone I, Palma L, Cantore G, Fortuna A. Cerebral oligodendroglioma: prognostic factors and life history. Neurosurgery. 1994;35:1018–1034. PMID: 7885546
21. Yeh SA, Lee TC, Chen HJ, Lui CC, Sun LM, Wang CJ, et al. Treatment outcomes and prognostic factors of patients with supratentorial low-grade oligodendroglioma. Int J Radiat Oncol Biol Phys. 2002;54:1405–1409. PMID: 12459363
22. Kiebert GM, Curran D, Aaronson NK, Bolla M, Menten J, Rutten EH, et al. EORTC Radiotherapy Co-operative GroupQuality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). Eur J Cancer. 1998;34:1902–1909. PMID: 10023313
23. Klein M, Heimans JJ, Aaronson NK, van der Ploeg HM, Grit J, Muller M, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360:1361–1368. PMID: 12423981
24. Feigenberg SJ, Amdur RJ, Morris CG, Mendenhall WM, Marcus RB Jr, Friedman WA. Oligodendroglioma: does deferring treatment compromise outcome? Am J Clin Oncol. 2003;26:e60–e66. PMID: 12796617
Fig. 1Overall survival rates are shown according to the treatment group-RT (n=43) versus OP (n=31). 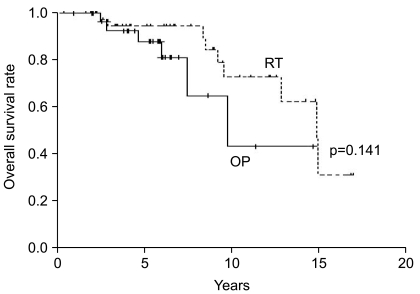
Fig. 2Progression-free survival rates are shown according to the treatment group-RT (n=43) versus OP (n=31). 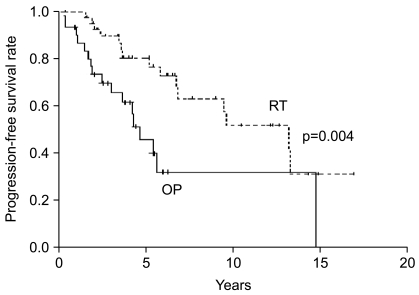
|
|
||||||||||||||||||||||||||||||||||||||