AbstractPurposeTo evaluate the effect of X-ray irradiation on apoptosis and change of expression of aquaporin 5 (AQP5) and transforming growth factor-β(TGF-β) in the rat submandibular gland (SMG).
Materials and MethodsSMGs of 120 male Sprague-Dawley rats were irradiated with a single X-ray dose (3, 10, 20, or 30 Gy). At the early and late post-irradiation phase, apoptosis was measured by the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) method, and expression of AQP5 and TGF-β was determined by immunohistochemical staining.
IntroductionRadiotherapy is a major treatment modality in the management of head and neck cancer, and the salivary glands of the patients are frequently included within the radiation field. The exposure of the salivary glands to high doses of radiation during radiotherapy results in salivary hypofunction, which may persist during the rest of the life of the patient leading to irreversible and distressing oral complaints. These consequences of treatment have a negative impact on the quality of life (1). Protection of the salivary glands against radiation damage is of great importance. Improvements in the technology for delivering therapeutic radiation such as three-dimensional conformal radiotherapy and intensity modulated radiotherapy have increasingly spared the salivary glands during head and neck irradiation. But, unfortunately, damage to the salivary glands still occurs, in part due to the continuing lack of understanding of the mechanisms of radiosensitivity of the salivary glands. Knowledge of these mechanisms would aid the development of strategies to protect the salivary glands from radiation.
The loss of salivary flow is observed soon after the administration of radiotherapy. The heightened radiosensitivity of acinar cells is an enigmatic behavior as these cells are nonmitotic, well-differentiated cells with a structure and function similar to exocrine pancreas cells, which are relatively radioresistant (2). Concerning the mechanisms of salivary gland radiosensitivity, some investigators hypothesized that leakage of lethal proteolytic enzymes through a disrupted membrane causes the immediate death of the serous cells (3). However, this hypothesis was rejected since radiation-induced cell death is not reduced by degranulation of the salivary gland acini prior to irradiation (4). Other investigators have suggested that the radiosensitivity of serous cells is derived from cellular DNA damage and subsequent cell death by apoptosis, also considered interphase cell death, since it is not linked to cell division (5). However, reports have described a lack of increase in apoptotic cells soon after irradiation (6). Currently, it is generally accepted that the early effects of radiation on salivary glands are due to dysfunction of non-removed cells at the level of the membranes and/or intracellular signaling (7).
In irradiated salivary glands, the mechanism of late radiation damage may differ from the mechanism of early damage. Although late tissue response to radiotherapy is important due to its progressive and irreversible character, the late effects of radiation on salivary glands have been studied less extensively. Generally, the mechanism of late radiation damage has been associated with a lack of proper cell renewal because of DNA damage to progenitor and stem cells (8), and by radiation damage to parenchymal cells, vascular endothelium and fibroblasts (9).
This study evaluated the effect of X-ray irradiation on apoptosis and the change in expression of two specific proteins that were presumed to have a relation to salivary dysfunction in the submandibular gland (SMG) of the rat. We examined the expression of aquaporin 5 (AQP5), a member of the major pathway for regulating water permeability in acinar cells (10), and transforming growth factor β(TGF-β), which has several important biological effects including regulation of cell death and extracellular matrix formation (11).
Materials and Methods1. AnimalsMale, 8~10-week-old Sprague Dawley rats (n=99) were divided randomly into five groups based on irradiation intensity: shamtreated controls (n=9), 3 Gy (n=18), 10 Gy (n=27), 20 Gy (n=18), and 30 Gy (n=18). All animal experiments were approved by the Institutional Animal Care and Use Committee at Seoul National University College of Medicine.
2. Irradiation procedurePrior to irradiation, rats were firmly immobilized in a custommade acrylic mold and anesthetized by an intraperitoneal injection of ketamine (Ketalar, Yuhan corporation, Korea, 60 mg/kg) and xylazine (Rompun, Bayer, US, 2.5 mg/kg). and were firmly immobilized in a custom-made acrylic mold. Irradiation was carried out with 6 MV X-rays from a Varian 21 EX linac linear accelerator (Varian Medical Systems, Palo Alto, CA) at a dose rate of 300 cGy/min. The irradiation field was circular with a diameter of 25 cm and six rats were irradiated simultaneously. The rats were locally irradiated in the region of the head and neck with a single dose of 3, 10, 20, or 30 Gy. Sham-treated control rats were subjected to the same procedure but were not irradiated.
3. Tissue preparationRats were sacrificed at the early post-irradiation phase (1 and 8 h, and 1, 2, 3 and 5 days after irradiation) and late post-irradiation phase (10, 20, 30 and 60 days after irradiation). Three animals per group were sacrificed at each time point in each irradiated groups; one animal was sacrificed at each time in the control group. The SMGs in all irradiated groups were analyzed up to 5 days after irradiation. Only the 10 Gy irradiated rats were analyzed from 10 days after irradiation. Small pieces (0.2~1.0 mm3) were separated from resected glands for electron microscopy. SMGs were fixed in 4% paraformaldehyde immediately after removal. After a minimum fixation of 48 h, the tissue was trimmed and was processed by standard paraffin-embedding methods.
4. Morphological and morphometrical analysesSections 4 µm in thickness were cut and deparaffinized, and the sections were stained with hematoxylin and eosin (H & E) to obtain conventional histological sections. For morphological analysis, the sections were coded and were randomly examined by the use of light microscopy with a model BX51 microscope (Olympus, Japan). For each examination, after the entire section was examined, 10 representative non-contiguous and non-overlapping fields were systematically selected and analyzed. After an initial qualitative assessment of the morphological changes, the number of acinar and granular convoluted tubule (GCT) cells were scored in five randomly chosen fields per section (field size: 145×215 µm2 at ×400 magnification) and the number of scored cells was averaged for each rat. The values were expressed as a percentage of the control value and the number of scored cells for a sham-treated control rat was set to 100%.
5. Apoptosis assayTo detect apoptotic cells, the terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) method was performed using an ApopTaq peroxidase In Situ apoptosis detection kit (Oncor, Gaithersburg, MD). Sections were deparaffinized, rehydrated in a decreasingly graded alcohol series, and incubated with proteinase K. Endogenous peroxidase in the sections was inhibited with 3% hydrogen peroxide, and slides were incubated at 37℃ for l h with terminal deoxynucleotidyl transferase (TdT) to catalyze the addition of digoxigenin-labeled nucleotides to the 3'-OH ends of fragmented DNA. Next, slides were incubated with horseradish peroxidase (HRP)-conjugated antidigoxigenin antibodies, and DNA fragmentation was detected by staining with 3'-3'diaminobenzidine (DAB). Finally, sections were counterstained with hematoxylin. Samples from negative controls omitted the addition of TdT. The sections stained by the use of the TUNEL assay were randomly chosen from each animal. The number of acinar, GCT and striated duct (SD) cells were scored. Approximately 1,000 cells from each cell population were counted at a magnification of ×400 using a BX51 light microscope (Olympus) and the percentage of TUNEL positive cells was calculated. The level of apoptosis for each group was obtained by averaging the percentages of all of the rats.
6. Electron microscopySmall pieces of tissue (0.2~1.0 mm3) were fixed with 2% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.2) at room temperature. These samples were postfixed with 1% OsO4 at 4℃ for 1 h, dehydrated and embedded in epoxy resin. Ultra-thin sections were observed using a JEM-100CX transmission electron microscope (JOEL, Japan).
7. Protein expressionImmunohistochemistry was performed to detect the expression of AQP5 and TGF-β. Analysis was performed only for 10 Gy- and 30 Gy-irradiated animals. The primary antibodies used were rabbit anti-rat AQP5 (2 µL/mL, Chemicon, Temecula, CA) and anti-mouse TGF-β(1 mg/mL, Chemicon). After dewaxing, rehydration and blocking of endogenous peroxidase, slides were subjected to microwave antigen retrieval (800 W, 3×5 min) in 0.01 M sodium citrate buffer (pH 6.0) to enhance staining. Primary antibody diluted 1 : 200 was applied overnight in a humidified chamber at 4℃, followed by the addition of a 1 : 200 dilution of rabbit-anti-mouse secondary antibody and streptavidin-horseradish peroxidase for 30 min each. The site of immunoreaction was made visible by DAB in the presence of hydrogen peroxide. Sections were finally counterstained with hematoxylin. The sections were randomly evaluated by a certified histologist who was blinded as to the origin of all the sections. After an initial qualitative assessment of the morphological changes, cells that showed definite staining after exposure to the antibodies were evaluated. To evaluate the degree of reduced protein expression on a per cell basis, a semiquantitative score was expressed as a percentage of the control value, and the number of scored cells for a sham-treated control rat was set to 100%. The median score of the measurement from the 10 fields was taken for each rat, and the median score of for each group was obtained.
8. Data and statistical analysesData are expressed as means±SEM. The comparison between experimental and control data was determined by use of two-way analysis of variance, followed by Turkey's honestly significant difference post hoc test, with a value of p<0.05 as statistically significant. SPSS software (SPSS, Chicago, IK) was used for evaluation.
Results1. Morphological and morphometrical change in irradiated ratsMorphological changes induced by SMG irradiation were assessed using conventional H & E staining. The SMG of the rat is composed of acinar, GCT, SD, intercalated duct (ID) and excretory duct (ED) cells. One hour post-irradiation, mild vacuolization of the acinar cells and some pyknotic nuclei in the acinar and GCT cells were observed. At day 3 post-irradiation, the salivary glands showed increased numbers of cytoplasmic vacuoles in the acinar cells and some degenerative changes that included frequent hyperstaining in the mucosal acini, hypostaining in the nuclei, edematic alteration and inter-acinar spaces with unclear outlines of the acini. At day 5 post-irradiation, SMGs showed histopathological changes that were similar but less prominent than those observed at day 3. These degenerative changes were detected more frequently at a high dose and a dose-related effect was evident. Tissue regeneration, evident as the re-appearance of normal histologic characteristics, was detected at day 10 post-irradiation. However, signs of late destruction in the irradiated glands including strong vacuolization, presence of many pyknotic nuclei and lysis of acinar cells were observed after day 30 post-irradiation. Large numbers of GCT and SD epithelial cells were degenerated and desquamated, and some duct cells were lysed. Interstitial edema and a slight elevation of fibrotic tissue were observed (Fig. 1). Based on morphometric analyses, irradiation did not induce changes in the number of acinar and GCT cells at any dose in early post-irradiation phase. However, the number of GCT and acinar cells decreased at late postirradiation phase (day 30 and 60, respectively; Fig. 2).
2. Apoptosis in irradiated ratsIn the SMGs of control rats, apoptosis was scant in acinar, GCT and SD cells. During the early post-irradiation phase, slightly increased apoptotic indices were observed in all cell types at all radiation doses, but not more than 5% of the cells became apoptotic even after a single dose as high as 30 Gy. At the late post-irradiation phase, an increased number of apoptotic cells were seen in irradiated rats. There were differences in the apoptotic percentage of acinar cells, but most apoptosis were found in GCT and SD cells (Fig. 3A, B). Electron microscopy also showed the apoptotic cells with a particular chromatin margination and condensation from irradiated animals. More apoptotic cells were observed at days 30 and 60 post-irradiation, and apoptotic nuclei were frequent in GCT and SD cells (Fig. 3C).
3. AQP5 a nd TGF-β expression in irradiated ratsLight microscopic examination of SMGs of control rats detected AQP5 expression in the apical membrane. AQP5 expression in SMGs of irradiated rats was similar to the controls for up to 2 days after exposure to X-rays. But, by day 5 post-irradiation, declined AQP5 expression was evident. The loss of AQP5 expression in 10 Gy-irradiated rats was comparable to the loss of AQP5 expression in 30 Gy-irradiated rats. Loss of AQP5 expression was observed throughout the late post irradiation phase, and was more pronounced than in the early post irradiation phase. In control SMGs, GCT, SD and ED cells had intense TGF-β expression. In contrast, no TGF-β expression was observed in acinar cells, ID cells or connective tissue. In most GCT cells, portions of the apical cytoplasm representing the secretory granules were more intensely immunoreactive than the rest of the cytoplasm. In SD and ED cells, TGF-β expression was diffuse throughout the entire cytoplasm, with substantially higher intensity in the apical portion. After irradiation, the pattern of SMG TGF-β expression was the same, but loss of TGF-β expression were observed in cells from irradiated animals. The loss was not conspicuous during the early irradiation phase. However, there was a statistically significant loss of TGF-β expression in cells from the irradiated groups at the late irradiation phase (Fig. 4, Table 1).
DiscussionExposure of salivary glands to ionizing radiation results in salivary hypofunction. Nagler et al. (12) described the effects of X-ray irradiation on salivary secretion over a 1 year period. Following irradiation with 15 Gy, secretion from the parotid and SMG continued to decrease and both glands exhibited a profound functional loss 76% for the parotid gland and 62% for the SMG at 12 months post-irradiation. In a report by Coppes et al. (7), the time kinetics of damage expression in rat SMG distinguished four phases within a time span of 0~240 days according to a change in salivary gland flow rate, amylase secretion and acinar cell number following local irradiation with 15 Gy of X-rays: acute (0~10 days), early (10~60 days), intermediate (60~120 days) and late (120~240 days). Although we did not examine salivary secretion in the irradiated rats, we consider our results to be similar to the findings reported by Coppes et al. (7), with the presently-described early and late post-irradiation phase corresponding to the acute and early phase, respectively. We observed a constant number of acinar and GCT cells in the early post-irradiation phase, while a loss of acinar and GCT cells occurred in the late post-irradiation phase. These results are consistent with the previous report (7).
Enhanced apoptosis of acinar cells is hypothesized to be one of the major causes of salivary gland impairment and can be induced with DNA damaging agents such as radiation. Early radiation damage in the salivary glands may reflect apoptotic cell death induced by exposure to radiation (5). This suggestion is supported by a more recent report that apoptosis plays a major role only during acute post-irradiation damage (13). However, we observed that radiation-induced apoptosis in the rat SMG occurred in both the early and late post-irradiation phases. Our findings are consistent with previously published results describing only a small level of apoptosis (<2%) after doses ranging from 5~15 Gy in rats at 1~3 days after irradiation (6) with a significantly higher apoptotic index in irradiated mice being apparent during late post-irradiation phase (14).
In addition, we observed that radiation markedly induced apoptosis of ductal cells of the salivary gland, implicating irradiation in salivary gland dysfunction during the late post-irradiation phase. However, it is also possible that radiation-induced apoptosis of ductal cells may have no relation with salivary gland dysfunction. Radiation may mainly affect acinar cells rather than ductal cells, since ductal cells are more radioresistant than acinar cells (15). In the latter study, exposure of salivary glands to radiation resulted in a dramatic reduction of saliva secretion but not a reduction of total solute and protein secretion. Further investigation is necessary to determine the effect on salivary gland function of the marked apoptosis of ductal cells induced by exposure to radiation.
AQP5 water channel proteins play important roles in saliva secretion (10) and may act as part of an apical water pathway during stimulated fluid secretion (16). This is the major pathway for regulating water permeability in acinar cells, and determines the flow rate and ionic composition of secreted saliva. Irradiation of rat SMG down-regulates the water channels in acinar cells accompanied with a significant loss of plasma membrane-bound AQP5 (15). We also observed a significant post-irradiation loss of AQP5 expression in acinar cell membrane at not only the early post-irradiation phase (5 days after irradiation) but also at the late post-irradiation phase. These results indicate that the loss of AQP5 stimulated by radiation may be responsible for salivary dysfunction in the late post-irradiation phase, as in the early post-irradiation phase.
TGF-β is a cytokine that is strongly involved in late radiation morbidity (17). Increased expression of TGF-β in response to radiation is important in the pathogenesis of radiation-induced fibrotic injury of the liver or lung (18). Changes in plasma TGF-β levels during radiotherapy have been reported as a predictor of the risk of developing radiation pneumonitis in humans (19). Whereas the expression of TGF-β in the evolution of radiation-induced lung injury has been well-studied (20), there are few reports concerning radiation-induced changes of TGF-β expression in the salivary glands.
A previous investigation of the correlation between the plasma TGF-β level and late morbidity including xerostomia in head and neck cancer patients treated with radiotherapy did not include histological examinations (21). Therefore, we examined the effect of X-ray irradiation on the changes of expression of TGF-β in rat SMG. Similar to a previous study (22), light microscopic observation of control SMG showed that the cells of the GCT, SD and ED were intensely immunoreactive for TGF-β. After irradiation, we observed that ductal cell TGF-β immunoreactivity decreased from day 10 post-irradiation and disappeared at day 30. Fibrotic tissues in the interstitial space were observed at the late post irradiation phase, but there was no expression of TGF-β in the interstitial space. These results are completely different from observations obtained in irradiated lung and indicate that fibrosis of the salivary gland is not related to the expression of TGF-β.
Parasymphathetic stimulation evokes both massive fluid secretion and increasing paracellular permeability in SMG acini (22-24) and salivation changes are related to permeability changes (24,25). Mukunoki et al. (22) observed TGF-β receptors distributed in the lateral and basal membrane of acinar cells, and a change of paracellular transporter by TGF-β. They suggested that the assembly of intercellular junctions in acinar cells may be affected by TGF-β-induced paracrine action of the GCT cells. It is difficult to explain the relationship between secreted TGF-β in GCT cells and the fluid secretion of acinar cells. An intercellular junction that is affected by TGF-β-induced paracrine action of GCT cells might regulate fluid secretion in acinar cells. Furthermore, radiation-induced loss of TGF-β might influence fluid secretion in acinar cells. However, it is unclear whether the rat SMG could be affected by the loss of TGF-β, since the role of TGF-β in the SMG is unknown.
ConclusionThe collective data supports the suggestion that apoptosis develops relatively late after X-ray irradiation in the rat SMG and contributes to the destruction of ductal cells. X-ray irradiation of the rat SMG induces the loss of AQP5 expression in acinar cells and the loss of TGF-β expression in ductal cells.
NotesSupported by grant No. 0620270 from the National R&D Program for Cancer Control, Ministry of Health Family & Welfare, Republic of Korea, and by Seoul National University Hospital Grant No. 04-2006-087 NotesPresented at the 34th annual meeting of Korean Cancer Association and the ESTRO 27, Göteborg, September 14~18, 2008. References1. Taylor JC, Terrell JE, Ronis DL, Fowler KE, Bishop C, Lambert MT, et al. Disability in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:764–769. PMID: 15210560
2. Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–1194. PMID: 15990024
3. Abok K, Brunk U, Jung B, Ericsson J. Morphologic and histochemical studies on the differing radiosensitivity of ductular and acinar cells of the rat submandibular gland. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:443–460. PMID: 6145251
4. Peter B, Van Waarde MA, Vissink A, s-Gravenmade EJ, Konings AW. The role of secretory granules in the radiosensitivity of rat salivary gland acini--a morphological study. Radiat Res. 1994;140:419–428. PMID: 7972696
5. Stephens LC, King GK, Peters LJ, Ang KK, Schultheiss TE, Jardine JH. Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death. Am J Pathol. 1986;124:469–478. PMID: 3766705
6. Paardekooper GM, Cammelli S, Zeilstra LJ, Coppes RP, Konings AW. Radiation-induced apoptosis in relation to acute impairment of rat salivary gland function. Int J Radiat Biol. 1998;73:641–648. PMID: 9690682
7. Coppes RP, Zeilstra LJ, Kampinga HH, Konings AW. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br J Cancer. 2001;85:1055–1063. PMID: 11592779
8. Zeilstra LJ, Vissink A, Konings AW, Coppes RP. Radiation induced cell loss in rat submandibular gland and its relation to gland function. Int J Radiat Biol. 2000;76:419–429. PMID: 10757322
9. Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223–231. PMID: 11730991
10. He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch. 1997;433:260–268. PMID: 9064641
11. Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. PMID: 11841535
12. Nagler RM. The enigmatic mechanism of irradiation-induced damage to the major salivary glands. Oral Dis. 2002;8:141–146. PMID: 12108758
13. Guchelaar HJ, Vermes A, Meerwaldt JH. Radiation-induced xerostomia: pathophysiology, clinical course and supportive treatment. Support Care Cancer. 1997;5:281–288. PMID: 9257424
14. Muhvic-Urek M, Bralic M, Curic S, Pezelj-Ribaric S, Borcic J, Tomac J. Apoptosis after Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse. Physiol Res. 2006;55:89–95. PMID: 15857161
15. Li Z, Zhao D, Gong B, Xu Y, Sun H, Yang B, et al. Decreased saliva secretion and down-regulation of AQP5 in submandibular gland in irradiated rats. Radiat Res. 2006;165:678–687. PMID: 16802868
16. Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;276:23413–23420. PMID: 11290736
17. Yi ES, Bedoya A, Lee H, Chin E, Kim SJ, Danielpour D, et al. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996;20:339–352. PMID: 8872498
18. Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys. 1992;24:93–101. PMID: 1512168
19. Anscher MS, Murase T, Prescott DM, Marks LB, Reisenbichler H, Bentel GC, et al. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–676. PMID: 7928499
20. Flanders KC, Burmester JK. Medical applications of transforming growth factor-beta. Clin Med Res. 2003;1:13–20. PMID: 15931280
21. Feltl D, Zavadova E, Pala M, Hozak P. Post-treatment plasma transforming growth factor beta 1 (TGF-beta1) level predicts for late morbidity in patients with advanced head and neck cancer. Neoplasma. 2005;52:393–397. PMID: 16151583
22. Mukunoki H, Uchihashi K, Nishikawa Y. Effect of TGF-β on paracellulat transport in the rat submandibular gland acini. J Osaka Dent Univ. 2004;38:41–52.
23. Higuchi H. Regulation of paracellular barrier function in mouse submandibular gland acini. J Osaka Dent Univ. 2001;35:13–25.
24. Takai N, Uchihashi K, Miyao H, Murakami H, Yoshida Y. Chorda-evoked opening of tight junctions in rat submandibular salivary acini demonstrated by microperoxidase. Arch Oral Biol. 1995;40:1077–1080. PMID: 8670027
25. Uchio T. Permeability changes in the rat submandibular gland with experimental sialothiasis. J Osaka Odont Soc. 1988;51:291–307.
Fig. 1Radiation-induced morphological changes in rat SMG. (A) Nonirradiated gland. The SMG parenchyma is composed mainly of acinar cells and duct cells. (a) Acini, (c) Granular convoluted tubules. (B) Day 3 after irradiation with 10 Gy. Increased numbers of cytoplasmic vacuoles (⇒) in acinar cells were found. (C) Day 60 after irradiation with 10 Gy. Numbers of GCT and SD cells were degenerated and the cells desquamated (→), and some duct cells were dissolved. Sections were stained with H & E. Magnification is ×400. 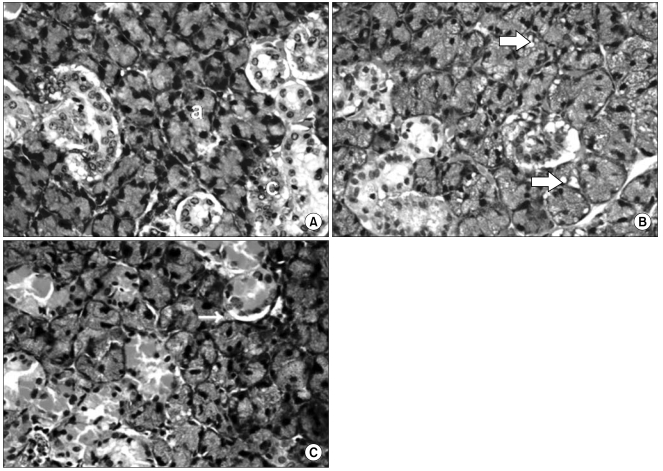
Fig. 2Morphometrical change in GCT cells of irradiated rats at the late post-irradiation phase. *Statistically significant difference from sham-treated control (p<0.05). 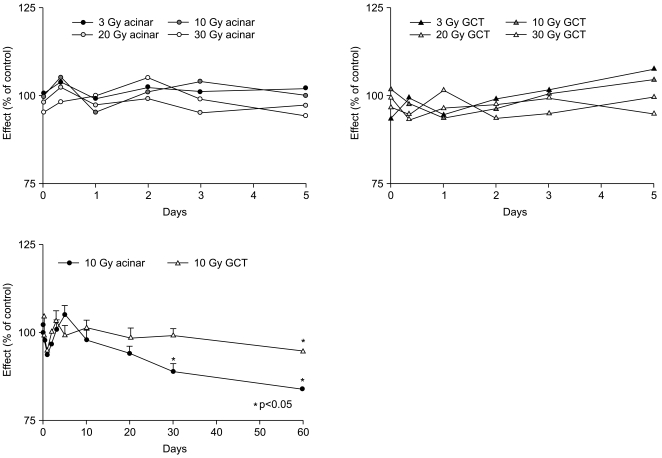
Fig. 3Apoptosis in submandibular gland of irradiated rats. (A) TUNEL assays in non-irradiated gland (a) and day 3 (b), day 10 (c), and day 30 (d) after irradiation with 10 Gy. (B) Time course of the percentage of apoptotic cells (%) in submandibular gland of irradiated rats. *Statistically significant difference from sham-treated control (p<0.05) (C) The finding in transmission electron microscopy. The apoptotic cells with nuclear chromatin condensation (⇒) and margination (←) were observed at day 30 and 60 after irradiation. Magnification is ×400. 
Fig. 4Altered AQP5 and TGF-β expression in irradiated rats. (A) Immunohistochemical staining of AQP5 in non-irradiated gland (a) and day 3 (b), day 10 (c), and day 30 (d) after irradiation with 10 Gy. A loss of AQP5 expression of acinar cells was observed at the late post irradiation phase. (B) Immunohistochemical staining of TGF-β in nonirradiated gland (a) and day 3 (b), day 10 (c), and day 30 (d) after irradiation with 10 Gy. A loss of TGF-β expression of ductal cells was observed at the late post irradiation phase. Magnification is ×400. 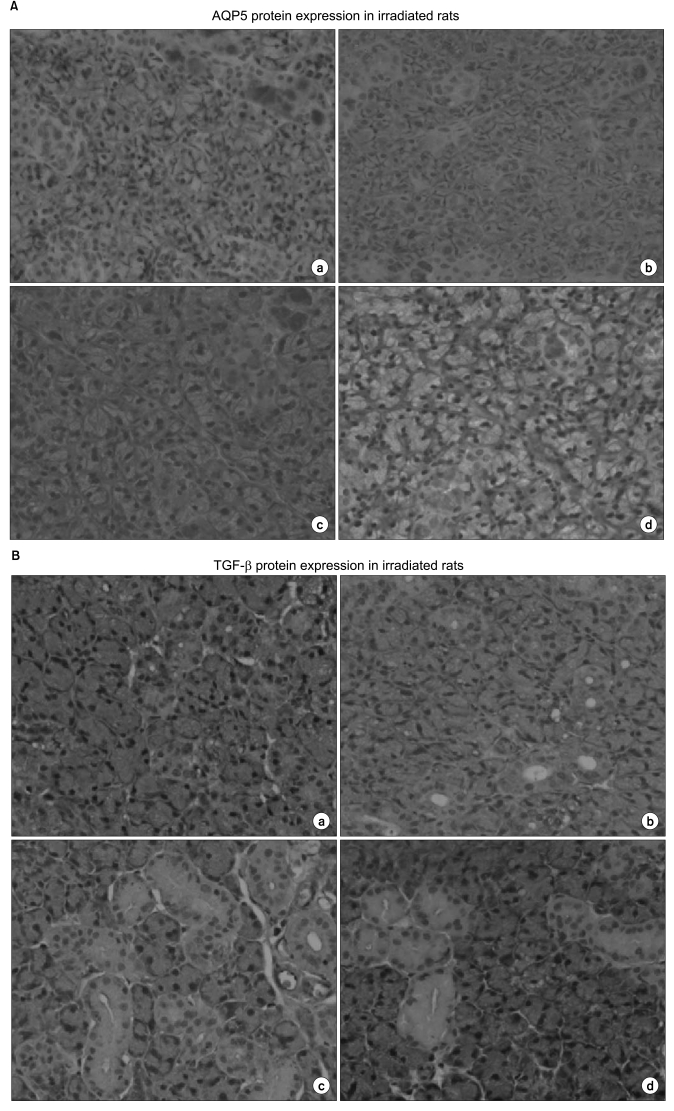
|
|
||||||||||||||||||||||||||||||||||||||