AbstractPurposeVascular endothelial growth factor (VEGF)-A, VEGF165b, interleukin (IL)-1β, and transforming growth factor (TGF)-β1 are known to influence tumor angiogenesis. Clinical implications of these cytokines need to be elucidated.
Materials and MethodsUsing clinical data and baseline serum samples of 140 consecutive patients with advanced non-small cell lung cancer who received platinum-based combination chemotherapy, we investigated the association among serum cytokine levels, treatment outcomes, as well as leukocyte and platelet counts.
ResultsThe median age of patients was 64 years (range, 26 to 86 years). The male to female ratio was 104:36. High TGF-β1 and IL-1β levels were associated with shorter progression-free survival, and high VEGF-A and IL-1β levels were associated with shorter overall survival in the univariate analysis. VEGF165b was not related to the treatment outcomes. Leukocytosis and thrombocytosis were associated with shorter overall survival. The multivariate analysis demonstrated that VEGF-A, IL-1β, and leukocytosis were significant prognostic factors (p=0.0497, p=0.047, and p<0.001, respectively). Leukocytosis was not associated with recent pneumonia (p=0.937) and correlated with VEGF-A (p<0.001) and TGF-β1 (p=0.020) levels.
IntroductionAngiogenesis, a process fundamental to tumor growth, is regulated by angiogenic cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β, and interleukin (IL)-1β [1,2].
Previously, a number of studies demonstrated that an elevated level of circulating VEGF-A was associated with tumor aggressiveness and unfavorable survival in patients with non-small cell lung cancer (NSCLC) [3]. In contrast, VEGF165b, a splice variant of VEGF, inhibited VEGF-mediated angiogenesis. The expression of VEGF165b was associated with slower tumor growth in vivo and benign tissue expressed a higher level of VEGF165b than the malignant tissue [4]. However, there has been no report regarding serum VEGF165b as a predictive or prognostic factor in patients with cancer.
TGF-β can induce VEGF in the fibroblastic and epithelial cells in vivo and promote angiogenesis [5,6]. In patients with NSCLC, tumor TGF-β1 level was associated with angiogenesis, tumor progression, and prognosis [7]. In addition, TGF-β1 enhanced the lethal effects of DNA-damaging agents in a human lung-cancer cell line [8]. Postoperative plasma TGF-β1 levels in patients with operable breast cancer were significantly higher compared to healthy individuals [9]. Preoperative plasma TGF-β1 levels in patients with advanced breast cancer and prostate cancer are associated with metastasis and disease progression [10,11]. However, the clinical role of serum TGF-β1 level in patients with NSCLC has not yet been well established.
IL-1β, an inflammatory cytokine, was identified as an essential initiating trigger of VEGF-dependent angiogenesis [2]. Serum IL-1β level in patients with ovarian cancer was significantly higher compared with healthy controls, and significantly decreased after surgical intervention [12]. However, clinical implications of serum IL-1β in patients with NSCLC are still uncertain.
On the other hand, VEGF-A and TGF-β1 are stored in and released from leukocytes and platelets, as well as tumor cells [3,7,13-15]. IL-1β is also produced by the peripheral blood mononuclear cells, as well as tumor cells [2,16]. Serum VEGF-A level was correlated with leukocyte or platelet counts of the peripheral blood [17,18]. However, the association between serum TGF-β1 or IL-1β levels and leukocyte or platelet counts in cancer patients has not been studied previously.
In this study, we aim to elucidate clinical implications of these cytokines as predictive or prognostic markers, and to correlate among serum cytokine levels, baseline leukocyte or platelet counts, and treatment outcomes.
Materials and Methods1. Patient populationPatients with solid tumors, who were planned to receive chemotherapy, were asked to participate in the pharmacogenomic study at Seoul National University Hospital (IRB Registration No. H-0610-020-186). After obtaining informed consent, patients donated blood for research purposes, and were registered in the database. From this database, we selected patients with stage IIIB or IV NSCLC who were treated with platinum-based combination chemotherapy as a first-line treatment between September 2005 and September 2008. Patients with histologically confirmed NSCLC were eligible for this study. Platinum-based combination chemotherapy was defined as cisplatin or carboplatin, plus one of the following: taxane, gemcitabine, vinorelbine, and etoposide. Patients who received concurrent chemoradiation as a first-line treatment or who had any history of prior chemotherapy, including adjuvant therapy, were excluded.
2. Study objectivesThe main objective of this study was to evaluate the association between VEGF-A, VEGF165b, IL-1β, and TGF-β1 serum levels and treatment outcomes of patients. In addition, the correlation of serum cytokine levels with leukocyte or platelet counts was evaluated. The prognostic impact of leukocyte or platelet counts was also studied.
3. Acquisition of clinical dataData regarding patient demographics, pathologic classification, treatment response, progression-free survival (PFS), and overall survival (OS) were obtained via a medical record review. We also collected clinical data regarding leukocyte and platelet counts at the time of serum sampling, along with the history of infection requiring antibiotics treatment within 1 month prior to chemotherapy. Patients who had smoked more than 100 cigarettes during the lifetime were defined as smokers. Tumor histology and subtypes were classified by the World Health Organization (WHO) criteria [19]. The treatment response was evaluated using a computed tomography scan by the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.0 [20]. PFS was calculated from the initiation of chemotherapy to documented disease progression or death from any cause. OS was calculated from the start of chemotherapy to death from any cause.
4. Quantification of serum cytokinesSerum samples acquired before chemotherapy were used for this study. The blood samples were collected and allowed to clot before centrifugation. Serum was removed and stored in liquid nitrogen until used. To measure cytokine levels from the baseline serum samples, enzyme-linked immunosorbent assay (ELISA) was performed, using commercially available kits, following the manufacturer's instructions for the following markers: VEGF-A (R&D Systems, Minneapolis, MN), VEGF165b (R&D Systems), IL-1β (R&D Systems), and TGF-β1 (R&D Systems). To measure serum levels of latent complexes of TGF-β1, the activation procedure by acidification was performed before ELISA as the manufacturer's instructions.
The capture antibody was diluted to the working concentration in phosphate buffered saline without carrier protein. A 96-well microplate was coated with the diluted capture antibody. The plate was sealed and incubated overnight at room temperature. After incubation, each well was aspirated and washed with wash buffer three times. Each well was then blocked with block buffer and incubated at room temperature for 1 hour. Each well was aspirated and washed again with a wash buffer three times. When the plate was prepared, the samples or standards in an appropriate diluent were added to each well. The plate was covered with an adhesive strip, which was then incubated at room temperature according to the manufacturer's instructions. After incubation, each well was washed with a wash buffer three times. Then, the detection antibody was added to each well. The plate was covered with a new adhesive strip and incubated at room temperature. After incubation, each well was washed again with a wash buffer three times. The working dilution of streptavidin-HRP was added to each well. The plate was covered and incubated again at room temperature, avoiding any direct light. After incubation, each well was washed again with a wash buffer three times. A substrate solution was added to each well. Then, the plate was incubated again at room temperature, avoiding any direct light. After incubation, a stop solution was added to each well. The optical density of each well was determined using a microplate reader (Multiskan Ascent, MTX Lab Systems, Inc., Vienna, VA), which was set to 450 nm with wavelength correction at 540 nm. Using measured optical density values, the concentration of each well was calculated. The samples were assayed in duplicate.
5. Statistical analysisThe median PFS and OS were calculated using the Kaplan-Meier method. The univariate and multivariate analyses of categorical variables was performed using logistic regression. The univariate and multivariate analyses of risk factors for survival data were performed using the Cox proportional hazard model. Variables with a p-value in the univariate analysis of less than 0.05 were selected for the multivariate analysis. Mann-Whitney U test was used to compare two different groups, consisting of non-parametric continuous variables. To correlate between serum cytokine levels and leukocyte counts, the Pearson correlation was used. Two-sided p-values below 0.05 were considered statistically significant. SPSS ver. 17.0 (SPSS Inc., Chicago, IL) software was used for all the statistical analyses.
6. Ethical considerationsSigned informed consents for chemotherapy and blood sample collection for the pharmacogenomic study were obtained from all patients before treatment. The protocol of this study was reviewed and approved by the institutional review board of Seoul National University Hospital (IRB Registration No. H-0906-071-284) and conducted in accordance with the precepts established by Helsinki Declaration.
Results1. Patient characteristicsA total of 140 patients were enrolled in this study. The median age of patients was 64 years (range, 25 to 86 years). One hundred four patients (74.3%) were male. Ninety patients (64.3%) were previous or current smokers. Tumor histology was as follows: adenocarcinoma in 73 patients (52.1%), squamous cell carcinoma in 29 patients (20.7%), large cell neuroendocrine carcinoma in 4 patients (2.9%), and not otherwise specified cases in 34 patients (24.3%). Twentyfour patients (17.1%) were initially diagnosed with stage IIIB disease. Among the other 116 patients (82.9%) with initial stage IV, 109 patients initially had metastatic disease and 7 patients had recurrent disease after curative treatment. Eastern Cooperative Oncology Group performance status were grade 0 in 11 patients (7.9%), 1 in 107 patients (76.4%), and 2 in 22 patients (15.7%). Chemotherapy regimens were as follows: taxane plus platinum in 98 patients (70.0%), gemcitabine plus platinum in 41 patients (29.3%), and etoposide plus platinum in 1 patient (0.7%).
During a median follow-up of 20.5 months (range, 1.0 to 40.7 months), 125 patients (89.3%) experienced disease progression and 82 patients (58.6%) died. The treatment response could be evaluated in 132 patients. The response rate of platinum-based combination chemotherapy was 47.0%. The median PFS was 3.9 months (95% confidence interval [CI], 3.0 to 4.7 months). The median OS was 13.0 months (95% CI, 9.7 to 16.3 months).
2. Serum cytokine levels and treatment resultsThe median VEGF-A level was 660 pg/mL (range, 49 to 3,722 pg/mL). VEGF165b levels were detectable (>15.6 pg/mL) by ELISA in 17 samples (12.1%). The median TGF-β1 level was 11,180 pg/mL (range, 511 to 41,210 pg/mL). IL-1β levels were detectable (>1.56 pg/mL) by ELISA in 19 samples (13.6%). The associations among serum cytokine levels were not statistically significant (data not shown).
The results of the univariate analysis are shown in Table 1. Smoking history was a significant factor for OS (p=0.020). Histology was a significant factor for the treatment response (p=0.038) and OS (p=0.013). The performance status was a significant factor for PFS (p<0.001) and OS (p=0.001). The association between cytokine levels and treatment responses was not significant. Patients with high VEGF-A levels (≥1,000 pg/mL, n=41) had shorter OS (median, 8.2 months [95% CI, 4.6 to 11.8 months] vs. 13.8 months [95% CI, 8.6 to 19.0 months]; p=0.035). VEGF165b was not associated with clinical outcomes of patients. High TGF-β1 levels (≥10,000 pg/mL, n=77) were associated with shorter PFS (median, 3.1 months [95% CI, 2.4 to 3.8 months] vs. 4.9 months [95% CI, 4.0-5.8 months]; p=0.025). High IL-1β levels (≥3.00 pg/mL, n=10) were associated with shorter PFS (median, 3.5 months [95% CI, 1.9 to 5.1 months] vs. 4.2 months [95% CI, 3.2 to 5.3 months]; p=0.046) and OS (median, 7.1 months [95% CI, 4.5 to 9.7 months] vs. 13.5 months [95% CI, 9.9 to 17.1 months]; p=0.030).
The results of the multivariate analysis are shown in Table 2. After adjustment for performance status, which was a significant variable in the univariate analysis, high TGF-β1 and IL-1β levels tended to be associated with a high risk of disease progression with a marginal significance (p=0.075 and p=0.070, respectively). VEGF-A (Fig. 1A) and IL-1β (Fig. 1B) were significant prognostic factors (p=0.0497 and p=0.047, respectively) after adjustment for smoking history, histology, and performance status.
3. Serum cytokine levels and baseline leukocyte or platelet countsSerum VEGF-A levels were correlated with leukocyte counts (r=0.422, p<0.001) (Fig. 2A) and platelet counts (r=0.462, p<0.001; data not shown). Serum TGF-β1 levels were also correlated with leukocyte counts (r=0.197, p=0.020) (Fig. 2B) and platelet counts (r=0.359, p<0.001; data not shown). However, serum VEGF165b and IL-1β levels were associated with neither leukocyte counts (p=0.071 and p=0.378, respectively) nor platelet counts (p=0.495 and p=0.816, respectively).
4. Baseline leukocyte or platelet counts and treatment resultsThe median leukocyte and platelet counts at the time of treatment were 7,505/mm3 (range, 3,940 to 55,560/mm3) and 278,000/mm3 (range, 68,000 to 692,000/mm3), respectively. The effects of the baseline leukocyte and platelet counts on OS are shown in Table 3. High leukocyte counts (≥9,000/mm3, n=39) and high platelet counts (≥400,000/mm3, n=20) were significant risk factors for OS in the univariate analysis. After adjustment for smoking history, histology, and performance status, the baseline leukocyte count was an unfavorable prognostic indicator (p<0.001) (Fig. 3).
5. History of infection and baseline leukocyte or platelet countsNineteen patients (13.6%) required antibiotics treatment for pneumonia within 1 month before the initiation of chemotherapy. No other infectious diseases developed. All patients had recovered from pneumonia, and antibiotics were stopped at the time of treatment. No significant association was found between the history of pneumonia and baseline leukocyte counts (p=0.937). The baseline platelet counts were also not significantly different according to the history of pneumonia (p=0.775).
DiscussionAs previously reported [3], serum VEGF-A level was a poor prognostic factor in this study. A previous study demonstrated that high tumor VEGF levels predicted a poor response to chemotherapy in patients with advanced breast cancer [21]. However, in the present study, serum VEGF-A level was associated with neither treatment response nor PFS.
On the other hand, VEGF-A exists as a number of isotypes, resulting from alternative pre-mRNA splicing. While most VEGF isotypes promotes angiogenesis, several anti-angiogenic VEGF isotypes have also been identified. Among them, the most dominant isotype is VEGF165b [4,22]. Since there has been no study regarding the predictive or prognostic role of serum VEGF165b level in patients with cancer, we focused on the clinical implication of VEGF165b in this study. However, we could not find any significant association between serum VEGF165b levels and treatment outcomes. Given that serum VEGF165b was not detectable by ELISA in most patients, the predictive or prognostic role of serum VEGF165b level is still inconclusive, and these associations should be evaluated again in further studies using more accurate quantification methods than ELISA.
Previously, tumor TGF-β1 level was reported to correlate with angiogenesis, tumor progression, and prognosis in patients with NSCLC [7]. In addition, TGF-β1 enhanced the lethal effects of DNA-damaging agents in a human lung-cancer cell line [8]. From these results, we hypothesized that serum TGF-β1 level might be associated with the chemotherapy response and prognosis. The univariate analysis demonstrated that high serum TGF-β1 level was significantly associated with shorter PFS, although this association was not statistically significant in the multivariate analysis. This subject needs to be evaluated again from future studies.
In this study, only 19 out of 140 patients (13.5%) had detectable (>1.56 pg/mL) serum IL-1β levels. Nevertheless, in the univariate analysis, we could find that a subset of patients with serum IL-1β levels higher than 3.00 pg/mL exhibited unfavorable clinical outcomes. This novel finding suggests that serum IL-1β level can be a useful predictive or prognostic marker in patients with advanced NSCLC. However, due to a limitation of the quantification method and marginal significance for PFS in the multivariate analysis, this result needs to be validated in further studies using a more accurate assay method and a larger patient cohort than those we used.
Normally, VEGF-A is stored in and released from leukocytes and platelets [3,13]. Previous studies showed that serum VEGF-A level was correlated with leukocyte or platelet counts of the peripheral blood, as well as tumor volume in patients with NSCLC [17,18]. During the coagulation process, activated platelets release VEGF in a rapid discharge reaction [23,24]. Our results also showed that serum VEGF-A levels were significantly correlated with both leukocyte and platelet counts. Besides VEGF-A, TGF-β1 are stored in and released from leukocytes and platelets [7,14,15]. IL-1β is also reported to be produced by leukocytes [2,16]. However, the association between serum TGF-β1 or IL-1β levels and leukocyte or platelet counts has not been well known. In the present study, serum TGF-β1 levels were significantly correlated with both leukocyte and platelet counts, while serum IL-1β was not.
Since serum VEGF-A level was a significant prognostic factor and significantly related to leukocyte and platelet counts, we evaluated the role of the baseline leukocyte and platelet counts as prognostic factors. Interestingly, leukocytosis and thrombocytosis were significantly associated with shorter OS in the univariate analysis, and the multivariate analysis showed that leukocytosis was an unfavorable prognostic factor. Since many other infectious causes, such as pneumonia, are able to induce leukocytosis, we compared the baseline leukocyte or platelet counts between patients with infection and those without. However, the baseline leukocyte or platelet counts were not significantly different between the two groups. In summary, high serum VEGF-A level was significantly associated with leukocytosis, and both leukocyte count and serum VEGF-A level were significant prognostic factors. In addition, these associations were independent from the previous history of infectious diseases.
A previous study demonstrated that the VEGF content of the isolated peripheral blood mononuclear cells and platelets was higher in cancer patients compared to healthy individuals [13]. In another study, serum VEGF levels were significantly higher than the matched plasma VEGF, and the difference between serum VEGF and plasma VEGF was also significantly higher in cancer patients with normal platelet levels than in normal controls [25]. Hence, cancer patients, compared to normal individuals, have more amount of VEGF, which was derived from leukocytes and platelets. These results suggest that non-specific inflammation induced by the tumor, irrespective of infection history, may result in leukocytosis, and that leukocytosis may contribute to increased production of angiogenic cytokines, such as VEGF-A and TGF-β1. As a result, the increased production of angiogenic cytokines may promote angiogenesis and tumor growth. If this vicious cycle can be blocked in cancer patients, tumor growth can be retarded or inhibited. Therefore, blocking this cycle could be a possible therapeutic target.
ConclusionSerum VEGF-A, TGF-β1, and IL-β1 levels, as well as leukocyte and platelet counts were significantly associated with clinical outcomes in patients with advanced NSCLC. In addition, leukocyte and platelet counts were significantly correlated with serum VEGF-A and TGF-β1 levels. Our results suggest that the increased production of angiogenic cytokines, such as VEGF-A and TGF-β1, from leukocytes and platelets induced by tumor-associated inflammation may promote angiogenesis and tumor growth, and may result in unfavorable treatment outcomes and prognosis in patients with advanced NSCLC.
AcknowledgmentsThis study was supported by a grant from the Seoul National University Research Fund (04-2009-0150) and the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (2010-0009563).
The authors wish to acknowledge the efforts of Ms. Hye Seon Ham and Ms. Su Jung Huh in Cancer Research Institute, Seoul National University, Seoul, Korea, and Ms. Hyun Yee Yoon in Laboratory of Protein Immunology, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea, for performing ELISA.
References1. Poon RT, Fan ST, Wong J. Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol. 2001;19:1207–1225. PMID: 11181687
2. Shchors K, Evan G. Tumor angiogenesis: cause or consequence of cancer? Cancer Res. 2007;67:7059–7061. PMID: 17671171
3. Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. PMID: 16360975
4. Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. PMID: 15520188
5. Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8:21–43. PMID: 9174661
6. Pertovaara L, Kaipainen A, Mustonen T, Orpana A, Ferrara N, Saksela O, et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. PMID: 8119973
7. Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–971. PMID: 11251948
8. Raynal S, Nocentini S, Croisy A, Lawrence DA, Jullien P. Transforming growth factor-beta1 enhances the lethal effects of DNA-damaging agents in a human lung-cancer cell line. Int J Cancer. 1997;72:356–361. PMID: 9219846
9. Chod J, Zavadova E, Halaska MJ, Strnad P, Fucikova T, Rob L. Preoperative transforming growth factor-beta 1 (TGF-beta 1) plasma levels in operable breast cancer patients. Eur J Gynaecol Oncol. 2008;29:613–616. PMID: 19115689
10. Shariat SF, Kattan MW, Traxel E, Andrews B, Zhu K, Wheeler TM, et al. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin Cancer Res. 2004;10:1992–1999. PMID: 15041717
11. Ivanovic V, Todorovic-Rakovic N, Demajo M, Neskovic-Konstantinovic Z, Subota V, Ivanisevic-Milovanovic O, et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer. 2003;39:454–461. PMID: 12751375
12. Zeisler H, Tempfer C, Joura EA, Sliutz G, Koelbl H, Wagner O, et al. Serum interleukin 1 in ovarian cancer patients. Eur J Cancer. 1998;34:931–933. PMID: 9797710
13. Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–491. PMID: 10100697
14. Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. PMID: 6602130
15. Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. PMID: 9597127
16. Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. PMID: 8001745
17. Choi JH, Kim HC, Lim HY, Nam DK, Kim HS, Yi JW, et al. Vascular endothelial growth factor in the serum of patients with non-small cell lung cancer: correlation with platelet and leukocyte counts. Lung Cancer. 2001;33:171–179. PMID: 11551412
18. Brattstrom D, Bergqvist M, Hesselius P, Larsson A, Lamberg K, Wernlund J, et al. Elevated preoperative serum levels of angiogenic cytokines correlate to larger primary tumours and poorer survival in non-small cell lung cancer patients. Lung Cancer. 2002;37:57–63. PMID: 12057868
19. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–97. PMID: 15898407
20. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. PMID: 10655437
21. Foekens JA, Peters HA, Grebenchtchikov N, Look MP, Meijer-van Gelder ME, Geurts-Moespot A, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. PMID: 11454684
22. Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: the vascular endothelial growth factor paradigm. Cancer Lett. 2007;249:133–142. PMID: 17027147
23. Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–668. PMID: 9012841
24. Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost. 1998;80:171–175. PMID: 9684805
25. Lee JK, Hong YJ, Han CJ, Hwang DY, Hong SI. Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol. 2000;17:149–152. PMID: 10853032
Fig. 1Overall survival according to serum vascular endothelial growth factor (VEGF)-A (A) and interleukin (IL)-1β levels (B). Serum VEGF-A (A) and IL-1β (B) levels were a significant prognostic factors (p=0.0497 and p=0.047, respectively) after adjustment for smoking history, histology, and performance status. 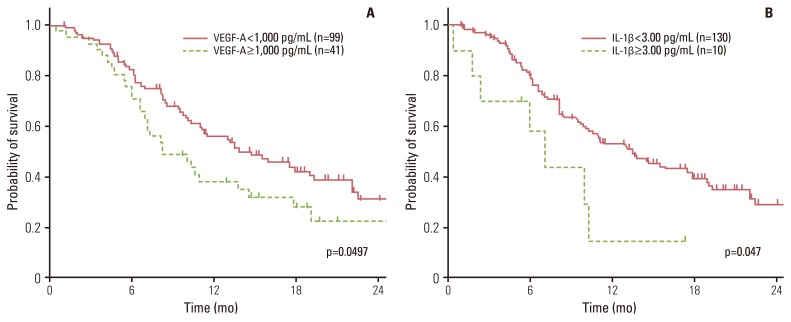
Fig. 2Correlation between serum vascular endothelial growth factor (VEGF)-A (A) or transforming growth factor (TGF)-β1 (B) levels and leukocyte counts. Serum VEGF-A (r=0.422, p<0.001) and TGF-β1 levels (r=0.197, p=0.020) were significantly correlated with leukocyte counts. 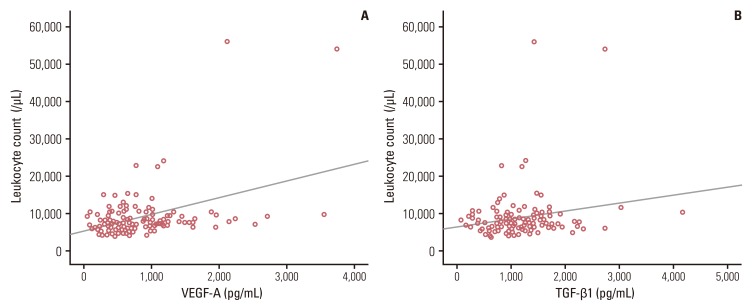
Fig. 3Overall survival according to leukocyte counts. Leukocyte count was an unfavorable prognostic factor (p<0.001). 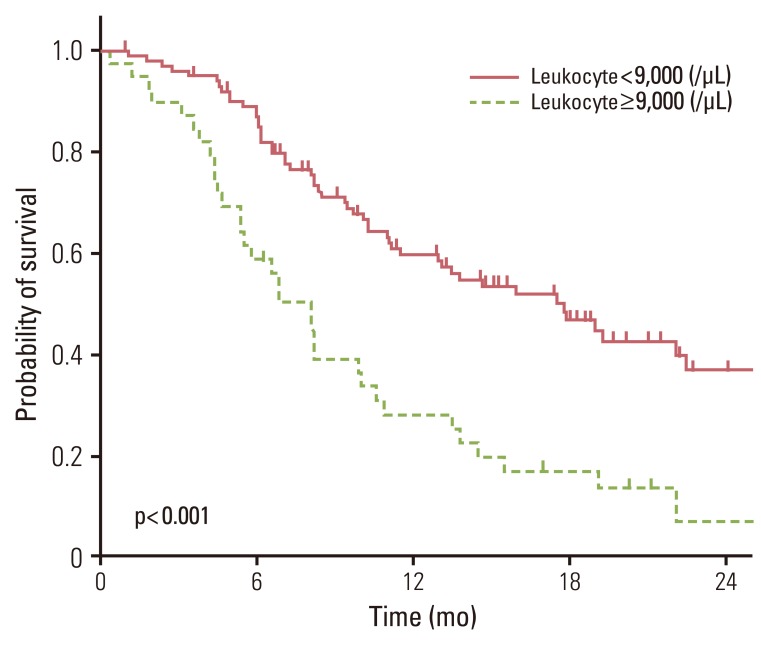
Table 1Univariate analysis for treatment response, progression-free survival, and overall survival
RR, relative risk; CI, confidence interval; HR, hazard ratio; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; NOS, not otherwise specified; ECOG PS, Eastern Cooperative Oncology Group performance status; TP, taxane+platinum; VEGF, vascular endothelial growth factor; TGF, transforming growth factor; IL, interleukin. a)Others include gemcitabine+platinum and etoposide+platinum. Table 2Multivariate analysis for progression-free survival and overall survival
Table 3Effects of baseline leukocyte or platelet counts on overall survival
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||